|
|
IV.A.4. (XIV.F.) |
Relative enthalpies of isomers - Comparison of 298.15K enthalpies (kJ mol-1)
Isomers of C5H7N
| index | Species | CAS number | Name | Relative experimental enthalpy (kJ mol-1) | sketch |
|---|---|---|---|---|---|
| a | C5H7N | 96548 | 1H-Pyrrole, 1-methyl- | 0.0 | 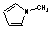 |
| b | C5H7N | 25899507 | (Z)-2-Pentenenitrile | 11.8 | 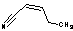 |
| c | C5H7N | 26294984 | (E)-2-Pentenenitrile | 16.6 |  |
| d | C5H7N | 16529661 | (E)-3-Pentenenitrile | 22.4 |  |
| e | C5H7N | 4426113 | Cyclobutanecarbonitrile | 44.2 | 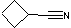 |
| semi-empirical | AM1 | 0.0 a -65.5 b -65.0 c -68.5 d |
|---|---|---|
| PM3 | 0.0 a 39.5 b 39.9 c 32.2 d 27.2 e |
|
| PM6 | 0.0 a | |
| MNDOd | 0.0 a -13.6 b -9.7 c -15.0 d |
|
| composite | G1 | 0.0 a 13.9 b 15.3 c 18.7 d 48.7 e |
| G2MP2 | 0.0 a 19.0 b 20.2 c 24.0 d 50.2 e |
|
| G2 | 0.0 a 17.2 b 18.3 c 22.2 d 48.8 e |
|
| G3 | 0.0 a 15.4 b 15.8 c 20.5 d 49.5 e |
|
| G3B3 | 0.0 a 15.0 b 15.3 c 19.1 d 48.3 e |
|
| G3MP2 | 0.0 a 16.3 b 16.9 c 21.1 d 52.0 e |
|
| G4 | 0.0 a 19.4 b 20.2 c 23.3 d 51.8 e |
|
| CBS-Q | NC |
|
| molecular mechanics | MM3 | -232.8 b -231.3 c 125.6 d |
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | aug-cc-pVDZ | aug-cc-pVTZ | cc-pV(T+d)Z | daug-cc-pVTZ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hartree fock | HF | 0.0 a 34.9 b 37.6 c 44.2 d -33.1 e |
0.0 a -13.2 b -10.4 c -5.9 d 19.8 e |
0.0 a -13.2 b -10.4 c -5.9 d 19.8 e |
0.0 a 0.8 b 2.4 c 6.9 d 48.5 e |
0.0 a -18.3 b -17.0 c -14.0 d 11.3 e |
0.0 a -15.9 b -14.8 c -12.0 d 14.8 e |
0.0 a -8.6 b -7.3 c -6.5 d 25.1 e |
0.0 a -22.4 b -21.4 c 10.9 e |
0.0 a -18.1 b -17.1 c -15.6 d 17.2 e |
0.0 a -17.2 b -16.3 c -13.0 d 15.7 e |
0.0 a -12.2 b -11.1 c -10.5 d 25.5 e |
0.0 a -18.5 b -17.2 c -17.0 d 18.3 e |
0.0 a -13.1 b -12.5 c -10.8 d 21.7 e |
NC NC NC NC |
0.0 a -12.2 b -11.6 c -10.7 d 20.1 e |
b c d e |
NC NC NC NC |
0.0 a -12.8 b -11.8 c -11.1 d 26.2 e |
| density functional | LSDA | NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
NC NC NC NC |
||||
| BLYP | 0.0 a 54.6 b 57.7 c 71.3 d 29.4 e |
0.0 a 1.2 b 3.7 c 14.5 d 39.4 e |
NC |
0.0 a 14.5 b 15.5 c 27.4 d 70.5 e |
0.0 a 14.1 b 15.4 c 26.4 d 58.4 e |
0.0 a 15.4 b 16.4 c 27.3 d 60.2 e |
0.0 a 17.4 b 18.4 c 27.4 d 64.7 e |
NC |
0.0 a 8.8 b 9.9 c 19.6 d 59.2 e |
0.0 a 19.7 b 20.8 c 32.3 d 66.3 e |
0.0 a 22.8 b 23.3 c 33.2 d 67.8 e |
0.0 a 12.0 b 13.0 c 21.8 d 65.9 e |
0.0 a 21.2 b 21.3 c 30.2 d |
0.0 a 12.0 b 13.0 c 21.8 d 65.9 e |
|||||
| B1B95 | 0.0 a 84.0 b 85.6 c 97.4 d 31.1 e |
0.0 a 32.9 b 33.9 c 44.6 d 44.9 e |
0.0 a 32.9 b 33.9 c 44.6 d 44.9 e |
0.0 a 48.5 b 48.2 c 58.8 d 76.0 e |
0.0 a 46.3 b 46.1 c 55.5 d 52.9 e |
0.0 a 47.2 b 46.8 c 56.2 d 62.3 e |
0.0 a 50.1 b 49.8 c 57.6 d 67.0 e |
0.0 a 38.8 b 38.3 c 47.0 d 56.6 e |
0.0 a 43.3 b 42.9 c 51.1 d 62.9 e |
0.0 a 52.6 b 52.2 c 61.9 d |
0.0 a 53.3 b 52.8 c 60.7 d 69.2 e |
0.0 a 50.3 b 49.7 c 57.1 d 63.3 e |
0.0 a 54.2 b 53.0 c 60.3 d |
0.0 a 50.3 b 49.7 c 57.1 d 63.3 e |
|||||
| B3LYP | 0.0 a 68.0 b 70.7 c 82.5 d 27.6 e |
0.0 a 12.8 b 15.1 c 24.6 d 42.8 e |
0.0 a 12.8 b 15.1 c 24.6 d 42.8 e |
0.0 a 27.7 b 28.4 c 38.7 d 74.2 e |
0.0 a 24.5 b 25.5 c 34.6 d 58.0 e |
0.0 a 26.1 b 26.8 c 35.9 d 60.3 e |
0.0 a 29.2 b 30.0 c 37.2 d 65.9 e |
b c d e |
0.0 a 20.4 b 21.1 c 28.9 d 60.5 e |
0.0 a 29.7 b 30.3 c 40.0 d 66.2 e |
0.0 a 26.2 b 27.0 c 33.5 d |
0.0 a 19.0 b 19.8 c 26.3 d 61.2 e |
0.0 a 32.5 b 32.7 c 40.7 d 67.6 e |
0.0 a 24.2 b 24.8 c 31.9 d 67.7 e |
0.0 a 32.1 b 32.0 c 39.2 d 66.0 e |
0.0 a 25.1 b 25.8 c 32.4 d -180.5 e |
0.0 a 24.2 b 24.8 c 31.9 d 67.7 e |
||
| B3LYPultrafine | 0.0 a 24.5 b 25.4 c 34.7 d |
0.0 a 25.1 b 25.7 c 32.4 d 68.5 e |
|||||||||||||||||
| B3PW91 | 0.0 a 79.6 b 82.1 c 93.6 d 28.7 e |
0.0 a 30.7 b 32.6 c 41.9 d 44.7 e |
0.0 a 30.7 b 32.6 c 41.9 d 44.7 e |
0.0 a 45.9 b 46.5 c 56.4 d 77.1 e |
0.0 a 43.8 b 44.5 c 53.5 d 61.4 e |
0.0 a 45.6 b 46.0 c 54.8 d 64.0 e |
0.0 a 48.9 b 49.4 c 56.6 d 69.0 e |
0.0 a 37.1 b 37.5 c 45.4 d 58.7 e |
0.0 a 41.8 b 42.2 c 49.7 d 65.1 e |
0.0 a 49.5 b 49.8 c 59.0 d 69.7 e |
0.0 a 51.5 b 51.6 c 59.1 d 70.9 e |
0.0 a 46.0 b 46.3 c 53.1 d 72.0 e |
0.0 a 51.7 b 51.3 c 58.2 d |
0.0 a 46.0 b 46.3 c 53.1 d 72.0 e |
|||||
| mPW1PW91 | 0.0 a 84.7 b 87.0 c 98.5 d 28.1 e |
0.0 a 36.6 b 38.4 c 48.0 d 40.8 e |
0.0 a 36.3 b 38.2 c 47.6 d 45.9 e |
0.0 a 51.1 b 51.6 c 61.5 d 78.0 e |
0.0 a 48.6 b 49.2 c 58.2 d 56.3 e |
0.0 a 50.5 b 50.8 c 59.5 d 59.0 e |
0.0 a 54.0 b 54.4 c 61.5 d 64.5 e |
0.0 a 42.1 b 42.4 c 54.0 e |
0.0 a 46.6 b 46.9 c 54.3 d 65.3 e |
0.0 a 53.8 b 54.1 c 63.2 d 69.5 e |
0.0 a 56.2 b 56.2 c 63.6 d 66.0 e |
0.0 a 50.8 b 50.9 c 57.6 d 72.3 e |
0.0 a 55.8 b 55.4 c 62.1 d |
0.0 a 50.8 b 50.9 c 57.6 d 72.3 e |
|||||
| M06-2X | b c d e |
0.0 a 39.0 b 39.5 c 47.4 d 56.7 e |
|||||||||||||||||
| PBEPBE | 0.0 a 80.1 b 82.3 c 96.9 d 38.0 e |
0.0 a 31.6 b 33.3 c 45.1 d 46.6 e |
0.0 a 31.6 b 33.3 c 45.1 d 46.6 e |
0.0 a 44.0 b 44.2 c 56.9 d 78.0 e |
0.0 a 45.6 b 46.1 c 57.9 d 66.7 e |
0.0 a 47.2 b 47.3 c 59.1 d 69.1 e |
0.0 a 49.3 b 49.6 c 59.5 d 72.9 e |
0.0 a 38.3 b 38.6 c 49.4 d 63.0 e |
0.0 a 43.0 b 43.3 c 53.6 d 69.3 e |
0.0 a 52.3 b 52.4 c 64.5 d 79.1 e |
0.0 a 54.1 b 53.9 c 64.3 d 76.3 e |
0.0 a 47.5 b 47.7 c 57.2 d 76.3 e |
0.0 a 53.3 b 52.8 c 62.3 d |
0.0 a 47.5 b 47.7 c 57.2 d 76.3 e |
|||||
| PBEPBEultrafine | 0.0 a 45.6 b 46.1 c 57.9 d |
||||||||||||||||||
| PBE1PBE | 0.0 a 52.5 b 52.9 c 62.1 d 62.7 e |
||||||||||||||||||
| HSEh1PBE | 0.0 a 37.5 b 39.0 c 49.3 d 47.9 e |
0.0 a 49.5 b 49.8 c 59.4 d |
0.0 a 54.8 b 55.0 c 62.7 d |
0.0 a 52.1 b 52.0 c 59.4 d 74.0 e |
|||||||||||||||
| TPSSh | 0.0 a 39.2 b 40.5 c 51.1 d 59.0 e |
0.0 a 44.6 b 45.7 c 54.5 d 66.8 e |
0.0 a 44.4 b 45.4 c 56.1 d 66.6 e |
0.0 a 41.9 b 42.7 c 51.1 d 69.7 e |
|||||||||||||||
| wB97X-D | 0.0 a 28.4 b 29.7 c 39.1 d 38.5 e |
0.0 a 39.7 b 40.0 c 48.7 d 50.8 e |
0.0 a 44.4 b 44.5 c 51.5 d 58.5 e |
0.0 a 36.5 b 36.5 c 44.0 d 54.0 e |
0.0 a 34.0 b 34.2 c 40.2 d 53.1 e |
0.0 a 44.4 b 44.5 c 51.5 d 58.5 e |
0.0 a 40.1 b 39.9 c 46.8 d 60.1 e |
0.0 a 40.3 b 40.1 c 46.6 d 59.9 e |
|||||||||||
| B97D3 | 0.0 a 7.9 b 10.9 c 23.7 d 40.8 e |
0.0 a 22.5 b 23.9 c 36.9 d 60.6 e |
0.0 a 27.0 b 28.3 c 39.4 d 67.8 e |
0.0 a 20.4 b 21.6 c 33.1 d 63.6 e |
0.0 a 25.2 b 26.5 c 36.7 d 70.5 e |
0.0 a 17.7 b 18.9 c 29.2 d 63.3 e |
0.0 a 23.2 b 24.4 c 35.1 d 69.6 e |
0.0 a 23.8 b 25.0 c 35.3 d 70.1 e |
|||||||||||
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | aug-cc-pVDZ | aug-cc-pVTZ | cc-pV(T+d)Z | daug-cc-pVTZ | ||
| Moller Plesset perturbation | MP2 | 0.0 a -62.7 b -59.2 c -55.1 d -92.5 e |
0.0 a -47.4 b -44.3 c -38.8 d -15.5 e |
0.0 a -47.4 b -44.3 c -38.8 d -15.5 e |
0.0 a -36.5 b -35.0 c -28.8 d 9.0 e |
0.0 a 15.9 b 18.0 c 24.3 d 33.1 e |
0.0 a 16.5 b 18.5 c 25.2 d 33.7 e |
0.0 a 14.4 b 16.8 c 21.9 d 29.7 e |
0.0 a 16.7 b 19.0 c 24.8 d 32.4 e |
0.0 a 33.6 b 36.1 c 43.7 d |
0.0 a 17.3 b 20.4 c 24.5 d 39.0 e |
0.0 a 20.7 b 22.5 c 28.4 d 38.1 e |
NC NC NC |
NC |
|||||
| MP2=FULL | NC |
NC |
NC |
NC |
0.0 a 17.5 b 19.6 c 26.0 d 34.1 e |
NC |
NC |
0.0 a 16.2 b 18.6 c 23.7 d 30.7 e |
0.0 a 18.7 b 20.9 c 26.7 d 33.5 e |
NC |
NC |
||||||||
| MP3 | 0.0 a 13.6 b 14.3 c 18.8 d 33.1 e |
||||||||||||||||||
| MP3=FULL | 0.0 a 14.9 b 15.6 c 20.2 d 33.9 e |
0.0 a 23.4 b 24.6 c 27.4 d 45.7 e |
|||||||||||||||||
| B2PLYP | 0.0 a 16.2 b 17.5 c 25.6 d 44.4 e |
0.0 a 22.7 b 23.7 c 30.4 d 58.9 e |
|||||||||||||||||
| Configuration interaction | CID | NC |
NC |
NC |
NC |
NC |
|||||||||||||
| CISD | NC |
NC |
NC |
NC |
NC |
||||||||||||||
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | aug-cc-pVDZ | aug-cc-pVTZ | cc-pV(T+d)Z | daug-cc-pVTZ | ||
| Quadratic configuration interaction | QCISD | NC |
NC |
NC |
NC |
NC |
NC |
NC |
NC |
NC |
|||||||||
| Coupled Cluster | CCD | NC |
NC |
NC |
NC |
NC |
NC |
NC |
NC |
||||||||||
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | aug-cc-pVDZ | aug-cc-pVTZ | cc-pV(T+d)Z | daug-cc-pVTZ |
| CEP-31G | CEP-31G* | CEP-121G | CEP-121G* | LANL2DZ | SDD | cc-pVTZ-PP | aug-cc-pVTZ-PP | Def2TZVPP | ||
|---|---|---|---|---|---|---|---|---|---|---|
| hartree fock | HF | 0.0 a 19.4 b 19.6 c 21.2 d 44.4 e |
0.0 a -2.2 b -2.1 c -1.8 d 7.4 e |
0.0 a 12.6 b 14.1 c 15.6 d 45.1 e |
0.0 a -16.9 b -15.3 c -15.8 d 7.3 e |
0.0 a 13.0 b 14.0 c 16.5 d 51.3 e |
0.0 a 12.8 b 13.8 c 16.4 d 50.5 e |
0.0 a -13.9 b -12.9 c -12.1 d 25.3 e |
||
| density functional | B1B95 | 0.0 a 76.5 b 74.2 c 83.5 d 78.0 e |
0.0 a 70.8 b 68.6 c 77.5 d 60.7 e |
|||||||
| B3LYP | 0.0 a 45.4 b 44.7 c 53.0 d 68.5 e |
0.0 a 36.6 b 36.3 c 44.0 d 51.1 e |
0.0 a 40.8 b 41.7 c 49.1 d 73.7 e |
0.0 a 24.6 b 26.0 c 32.3 d 52.8 e |
0.0 a 45.6 b 45.5 c 54.4 d 80.6 e |
0.0 a 45.5 b 45.4 c 54.2 d 80.5 e |
0.0 a 25.2 b 25.8 c 32.5 d 68.3 e |
|||
| PBEPBE | 0.0 a 48.4 b 48.5 c 57.7 d 76.8 e |
|||||||||
| Moller Plesset perturbation | MP2 | 0.0 a -22.1 b -23.4 c -18.4 d 1.1 e |
0.0 a 23.4 b 23.1 c 28.6 d 19.9 e |
0.0 a -21.4 b -20.0 c -16.4 d 9.9 e |
0.0 a 21.7 b 24.7 c 28.0 d 31.6 e |
0.0 a -26.6 b -24.7 c -20.6 d 9.4 e |
0.0 a -27.2 b -25.3 c -21.2 d 8.5 e |
0.0 a 36.3 b 38.2 c 44.0 d 55.4 e |
| 6-311+G(3df,2p) | cc-pVDZ | cc-pVTZ | aug-cc-pVDZ | cc-pV(T+d)Z | ||
|---|---|---|---|---|---|---|
| Moller Plesset perturbation | MP2FC// HF/6-31G* | 0.0 a -20.8 b -15.7 c -13.0 d |
0.0 a 3.3 b 9.9 c 26.5 d |
0.0 a 3.3 b 9.9 c 26.5 d |
||
| MP2FC// B3LYP/6-31G* | 0.0 a 24.0 b 25.4 c 31.5 d |
|||||
| MP2FC// MP2FC/6-31G* | NC |
0.0 a 29.4 b 31.3 c 36.4 d |
||||
| MP4// MP2/6-31G* | NC |
|||||
| Coupled Cluster | CCSD// MP2FC/6-31G* | NC |
||||
| CCSD(T)// MP2FC/6-31G* | NC |
For descriptions of the methods (AM1, HF, MP2, ...) and basis sets (3-21G, 3-21G*, 6-31G, ...) see the glossary in section I.C. Predefined means the basis set used is determined by the method.
gaw refers to the group additivity method implemeted in the NIST Chemistry Webbook.
See section III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.