|
|
IV.A.4. (XIV.F.) |
Relative enthalpies of isomers - Comparison of 298.15K enthalpies (kJ mol-1)
Isomers of CH3BO
| index | Species | CAS number | Name | Relative experimental enthalpy (kJ mol-1) | sketch |
|---|---|---|---|---|---|
| a | BH3CO | 13205442 | Borane carbonyl | 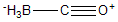 |
|
| b | CH3BO | 79723210 | Borane, methyloxo- | 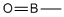 |
|
| c | CH2BOH | 422506716 | hydroxy(methylene)borane | 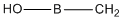 |
| semi-empirical | AM1 | 9.6 a |
|---|---|---|
| PM3 | 24.3 a | |
| PM6 | -21.1 a | |
| composite | G1 | NC NC NC |
| G2MP2 | NC NC NC |
|
| G2 | NC NC NC |
|
| G3 | NC NC NC |
|
| G3B3 | NC NC NC |
|
| G4 | NC NC NC |
|
| CBS-Q | NC NC NC |
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | cc-pVQZ | aug-cc-pVDZ | aug-cc-pVTZ | aug-cc-pVQZ | cc-pCVDZ | cc-pCVTZ | daug-cc-pVTZ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hartree fock | HF | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | NC NC |
|
| ROHF | NC | NC | ||||||||||||||||||||
| density functional | LSDA | NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC | NC | ||||
| BLYP | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||||
| B1B95 | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC |
NC | NC | |||||
| B3LYP | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||
| B3LYPultrafine | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | ||||||||||
| B3PW91 | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||||
| mPW1PW91 | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||||
| M06-2X | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||||
| PBEPBE | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||||
| PBEPBEultrafine | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | ||||||||||
| PBE1PBE | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||||
| HSEh1PBE | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||||
| TPSSh | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | ||||||||
| wB97X-D | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||
| B97D3 | NC NC |
NC | NC | |||||||||||||||||||
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | cc-pVQZ | aug-cc-pVDZ | aug-cc-pVTZ | aug-cc-pVQZ | cc-pCVDZ | cc-pCVTZ | daug-cc-pVTZ | ||
| Moller Plesset perturbation | MP2 | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC NC |
NC | NC | ||
| MP2=FULL | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||
| ROMP2 | NC | NC | ||||||||||||||||||||
| MP3 | NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC | NC | |||||||||||||||
| MP3=FULL | NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC | NC | ||||||
| MP4 | NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC | NC | ||||||||||||
| MP4=FULL | NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC |
NC NC NC |
NC NC NC |
NC | NC | |||||||||||||
| B2PLYP | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||||
| B2PLYP=FULL | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | |||||
| Configuration interaction | CID | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
||||||||||||||||
| CISD | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
|||||||||||||||||
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | cc-pVQZ | aug-cc-pVDZ | aug-cc-pVTZ | aug-cc-pVQZ | cc-pCVDZ | cc-pCVTZ | daug-cc-pVTZ | ||
| Quadratic configuration interaction | QCISD | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
|||||||
| QCISD(T) | NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC | NC | |||||||||||||
| QCISD(T)=FULL | NC NC NC |
NC NC NC |
NC NC NC |
NC NC |
NC NC |
NC NC NC |
NC NC NC |
NC |
NC | NC | ||||||||||||
| QCISD(TQ) | NC NC NC |
NC NC NC |
NC NC |
NC NC NC |
||||||||||||||||||
| QCISD(TQ)=FULL | NC NC NC |
NC NC NC |
NC NC NC |
|||||||||||||||||||
| Coupled Cluster | CCD | NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC | NC | |||||
| CCSD | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC |
NC NC |
NC NC |
NC | NC | ||||||||||||
| CCSD=FULL | NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC NC |
NC | NC | ||||||||||||
| CCSD(T) | NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC |
NC NC NC |
NC NC NC |
NC |
NC | NC | ||||||||||
| CCSD(T)=FULL | NC NC NC |
NC NC NC |
NC NC NC |
NC NC NC |
NC NC |
NC NC NC |
NC NC NC |
NC | NC | |||||||||||||
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | cc-pVQZ | aug-cc-pVDZ | aug-cc-pVTZ | aug-cc-pVQZ | cc-pCVDZ | cc-pCVTZ | daug-cc-pVTZ |
| CEP-31G | CEP-31G* | CEP-121G | CEP-121G* | LANL2DZ | SDD | cc-pVTZ-PP | aug-cc-pVTZ-PP | Def2TZVPP | ||
|---|---|---|---|---|---|---|---|---|---|---|
| hartree fock | HF | -67172.3 b -66978.4 c |
-67325.7 b -67109.0 c |
-67189.2 b -67004.7 c |
-67346.8 b -67139.5 c |
NC NC |
NC NC |
NC NC |
||
| density functional | B3LYP | -68805.5 b -68601.9 c |
-68904.0 b -68692.9 c |
-68824.2 b -68628.2 c |
-68929.7 b -68726.1 c |
NC NC |
NC NC |
NC NC |
||
| PBEPBE | NC NC |
|||||||||
| wB97X-D | -68807.3 b -68599.3 c |
-68907.1 b -68693.0 c |
-68825.6 b -68624.9 c |
-68931.2 b -68724.9 c |
NC NC |
NC NC |
||||
| Moller Plesset perturbation | MP2 | -67820.6 b -67580.4 c |
-68291.4 b -68050.2 c |
-67913.0 b -67682.9 c |
-68379.0 b -68147.4 c |
NC NC |
NC NC |
NC NC |
For descriptions of the methods (AM1, HF, MP2, ...) and basis sets (3-21G, 3-21G*, 6-31G, ...) see the glossary in section I.C. Predefined means the basis set used is determined by the method.
gaw refers to the group additivity method implemeted in the NIST Chemistry Webbook.
See section III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.