|
|
VII.C.4. |
Notes on Ionization Energies
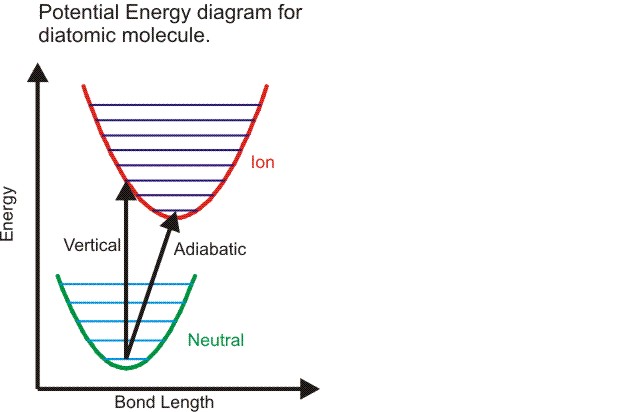
The difference between a vertical ionzation energy and adiabatic ionzation energy
The geometry of an ion may be different from the neutral molecule. The measured ionzation energy can refer to the vertical ionization energy, in which case the ion is in the same geometry as the neutral, or to the adiabatic ionzaiton energy, in which case the ion is in its lowest energy, relaxed geometry. This is illustrated in the figure. For a diatomic the only geometry change possible is the bond length. The figure shows an ion with a slightly longer bond length than the neutral. The harmonic potential energy surfaces are shown in green (neutral) and red (ion) with vibrational energy levels. The vertical ionzation energy is always greater than the adiabatic ionzation energy.