|
|
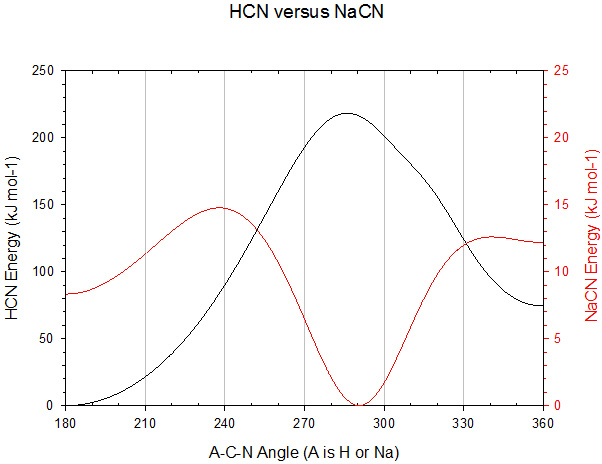
XXI.A. |
NaCN is not linear
Assuming similarity to H-C≡N (hydrogen cyanide), which is linear, it is reasonable to assume gas-phase Na-C≡N would be linear too. An isomer of H-C≡N exists, C≡N-H (hydrogen isocyanide, also linear) which is approximately 62 kJ mol-1 higher in energy. For Na-C≡N there is a very marginally stable structure for the C≡N-Na geometry. It is close to the same energy (within 10 kJ mol-1) of the Na-C≡N geometry. However the lowest energy (most stable) geometry is triangular with the Na almost perpendicular to the C≡N bond.