|
|
II.A.3. (XII.A.1.) |
Listing of experimental geometry data for CH3NO (nitrosomethane)
Rotational Constants (cm-1)
See section I.F.4 to change rotational constant units
| A | B | C |
|---|---|---|
| 2.04421 | 0.38202 | 0.34205 |
Calculated rotational constants for CH3NO (nitrosomethane).
Point Group Cs
Internal coordinates
(distances (r) in Å) (angles (a) in degrees) (dihedrals (d) in degrees)| Description | Value | Connectivity | Reference | Comment | |||
|---|---|---|---|---|---|---|---|
| Atom 1 | Atom 2 | Atom 3 | Atom 4 | ||||
| rNO | 1.211 | 2 | 3 | 1978Tur/Cox:533-559 | |||
| rCN | 1.482 | 1 | 2 | 1978Tur/Cox:533-559 | |||
| rCH | 1.094 | 1 | 4 | 1978Tur/Cox:533-559 | symmetric H | ||
| rCH | 1.092 | 1 | 5 | 1978Tur/Cox:533-559 | out of plane | ||
| aCNO | 113.16 | 1 | 2 | 3 | 1978Tur/Cox:533-559 | ||
| aHCN | 111.08 | 2 | 1 | 4 | 1978Tur/Cox:533-559 | ||
| aHCN | 107.26 | 2 | 1 | 5 | 1978Tur/Cox:533-559 | out of plane H | |
| aHCH | 109.27 | 5 | 1 | 6 | 1978Tur/Cox:533-559 | out of plane Hs | |
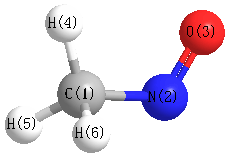
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type | Count |
|---|---|
| H-C | 3 |
| C-N | 1 |
| N=O | 1 |
| Atom | x (Å) | y (Å) | z (Å) |
|---|---|---|---|
| C1 | -1.1864 | -0.1800 | 0.0000 |
| N2 | 0.1245 | 0.5113 | 0.0000 |
| O3 | 1.0653 | -0.2516 | 0.0000 |
| H4 | -1.0582 | -1.2665 | 0.0000 |
| H5 | -1.7261 | 0.1489 | 0.8905 |
| H6 | -1.7261 | 0.1489 | -0.8905 |
Atom - Atom Distances (Å)
| C1 | N2 | O3 | H4 | H5 | H6 | |
|---|---|---|---|---|---|---|
| C1 | 1.4820 | 2.2528 | 1.0940 | 1.0920 | 1.0920 | |
| N2 | 1.4820 | 1.2112 | 2.1353 | 2.0854 | 2.0854 | |
| O3 | 2.2528 | 1.2112 | 2.3536 | 2.9572 | 2.9572 | |
| H4 | 1.0940 | 2.1353 | 2.3536 | 1.8007 | 1.8007 | |
| H5 | 1.0920 | 2.0854 | 2.9572 | 1.8007 | 1.7810 | |
| H6 | 1.0920 | 2.0854 | 2.9572 | 1.8007 | 1.7810 |
Calculated geometries for CH3NO (nitrosomethane).
References
By selecting the following links, you may be leaving NIST webspace. We have provided these links to other web sites because they may have information that would be of interest to you. No inferences should be drawn on account of other sites being referenced, or not, from this page. There may be other web sites that are more appropriate for your purpose. NIST does not necessarily endorse the views expressed, or concur with the facts presented on these sites. Further, NIST does not endorse any commercial products that may be mentioned on these sites. Please address comments about this page to cccbdb@nist.gov.
By selecting the following links, you may be leaving NIST webspace. We have provided these links to other web sites because they may have information that would be of interest to you. No inferences should be drawn on account of other sites being referenced, or not, from this page. There may be other web sites that are more appropriate for your purpose. NIST does not necessarily endorse the views expressed, or concur with the facts presented on these sites. Further, NIST does not endorse any commercial products that may be mentioned on these sites. Please address comments about this page to cccbdb@nist.gov.
| squib | reference | DOI |
|---|---|---|
| 1978Tur/Cox:533-559 | PH Turner, AP Cox "Microwave spectrum, structure, dipole moment and centrifugal distortion of nitrosomethane. Dipole moment of acetaldehyde" J. Chem. Soc., Faraday Trans. 2, 1978,74, 533-559 | 10.1039/F29787400533 |
Got a better number? Please email us at
cccbdb@nist.gov
| Browse | |
|---|---|
| Previous | Next |