|
|
IV.A.4. (XIV.F.) |
Relative enthalpies of isomers - Comparison of 298.15K enthalpies (kJ mol-1)
Isomers of C4H4
| index | Species | CAS number | Name | Relative experimental enthalpy (kJ mol-1) | sketch |
|---|---|---|---|---|---|
| a | C4H4 | 157391 | Tetrahedrane |  |
|
| b | C4H4 | 1120532 | cyclobutadiene |  |
|
| c | H2CCCCH2 | 2873509 | Butatriene | 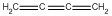 |
|
| d | C2H3CCH | 689974 | 1-Buten-3-yne | 0.0 | 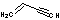 |
| semi-empirical | AM1 | 552.5 a 34.2 c 0.0 d |
|---|---|---|
| PM3 | 477.2 a 435.1 b 51.3 c 0.0 d |
|
| PM6 | 501.2 a | |
| composite | G1 | 256.1 a 143.7 b 28.8 c 0.0 d |
| G2MP2 | 242.9 a 139.2 b 28.0 c 0.0 d |
|
| G2 | 244.7 a 140.0 b 28.3 c 0.0 d |
|
| G3 | 251.1 a 143.9 b 27.2 c 0.0 d |
|
| G3B3 | 251.3 a 320.3 b 30.5 c 0.0 d |
|
| G3MP2 | 25.9 c 0.0 d |
|
| G4 | 244.5 a 316.5 b 30.0 c 0.0 d |
|
| CBS-Q | NC NC |
|
| molecular mechanics | MM3 | -65.6 c |
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | cc-pVQZ | aug-cc-pVDZ | aug-cc-pVTZ | aug-cc-pVQZ | cc-pV(T+d)Z | daug-cc-pVTZ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hartree fock | HF | 152.6 b 82.0 c 0.0 d |
219.9 b 42.8 c 0.0 d |
219.9 b 42.8 c 0.0 d |
208.2 b 41.4 c 0.0 d |
44.2 c 0.0 d |
172.9 b 44.3 c 0.0 d |
173.9 b 45.6 c 0.0 d |
178.7 b 45.5 c 0.0 d |
178.8 b 47.3 c 0.0 d |
175.6 b 42.8 c 0.0 d |
45.7 c 0.0 d |
183.5 b 47.1 c 0.0 d |
170.4 b 44.1 c 0.0 d |
178.8 b 46.1 c 0.0 d |
NC |
169.2 b 44.8 c 0.0 d |
177.9 b 46.0 c 0.0 d |
NC |
46.1 c 0.0 d |
45.9 c 0.0 d |
| density functional | LSDA | 80.7 b 11.8 c 0.0 d |
148.8 b -0.1 c 0.0 d |
148.8 b -0.1 c 0.0 d |
139.5 b -2.7 c 0.0 d |
114.1 b -5.6 c 0.0 d |
113.3 b -4.8 c 0.0 d |
115.5 b -2.1 c 0.0 d |
125.6 b -1.4 c 0.0 d |
125.0 b 0.5 c 0.0 d |
118.8 b -4.1 c 0.0 d |
NC |
110.9 b -1.4 c 0.0 d |
125.0 b 0.7 c 0.0 d |
112.2 b 0.9 c 0.0 d |
NC |
0.7 c 0.0 d |
||||
| BLYP | 114.5 b 15.2 c 0.0 d |
179.5 b 3.6 c 0.0 d |
179.5 b 3.6 c 0.0 d |
175.7 b 0.5 c 0.0 d |
309.1 b -2.5 c 0.0 d |
153.2 b -1.5 c 0.0 d |
156.1 b 1.4 c 0.0 d |
165.6 b 1.7 c 0.0 d |
165.1 b 3.7 c 0.0 d |
161.2 b -1.5 c 0.0 d |
NC |
152.1 b 2.2 c 0.0 d |
167.4 b 3.5 c 0.0 d |
4.7 c 0.0 d |
3.5 c 0.0 d |
||||||
| B1B95 | 104.0 b 28.6 c 0.0 d |
170.9 b 13.3 c 0.0 d |
170.9 b 0.0 d |
164.2 b 10.0 c 0.0 d |
130.4 b 9.1 c 0.0 d |
152.6 b 8.3 c 0.0 d |
137.1 b 10.7 c 0.0 d |
144.7 b 11.1 c 0.0 d |
144.2 b 12.8 c 0.0 d |
139.0 b 8.6 c 0.0 d |
NC |
131.9 b 10.6 c 0.0 d |
138.7 b 0.0 d |
127.9 b 0.0 d |
138.1 b 0.0 d |
0.0 d |
|||||
| B3LYP | 115.6 b 28.2 c 0.0 d |
182.3 b 11.9 c 0.0 d |
182.3 b 11.9 c 0.0 d |
175.7 b 9.1 c 0.0 d |
6.9 c 0.0 d |
150.4 b 7.6 c 0.0 d |
152.8 b 10.2 c 0.0 d |
162.1 b 10.6 c 0.0 d |
161.7 b 12.6 c 0.0 d |
157.9 b 7.4 c 0.0 d |
12.2 c 0.0 d |
165.0 b 12.4 c 0.0 d |
149.1 b 10.6 c 0.0 d |
163.5 b 12.2 c 0.0 d |
NC |
151.0 b 12.2 c 0.0 d |
162.9 b 12.5 c 0.0 d |
NC |
-124.5 c 0.0 d |
||
| B3LYPultrafine | 151.3 b 6.9 c 0.0 d |
NC |
NC |
NC NC |
NC |
162.9 b 12.5 c 0.0 d |
|||||||||||||||
| B3PW91 | 105.9 b -121.9 c 0.0 d |
167.8 b 11.7 c 0.0 d |
167.8 b 11.7 c 0.0 d |
161.7 b 8.7 c 0.0 d |
136.1 b 6.4 c 0.0 d |
135.0 b 7.1 c 0.0 d |
136.5 b 9.4 c 0.0 d |
144.3 b 9.7 c 0.0 d |
143.8 b 11.5 c 0.0 d |
140.4 b 7.3 c 0.0 d |
NC |
132.6 b 9.5 c 0.0 d |
145.0 b 11.0 c 0.0 d |
10.8 c 0.0 d |
0.0 d |
11.0 c 0.0 d |
|||||
| mPW1PW91 | 30.6 c 0.0 d |
14.0 c 0.0 d |
14.0 c 0.0 d |
11.0 c 0.0 d |
8.9 c 0.0 d |
9.5 c 0.0 d |
11.9 c 0.0 d |
12.0 c 0.0 d |
13.8 c 0.0 d |
9.7 c 0.0 d |
11.7 c 0.0 d |
13.3 c 0.0 d |
13.1 c 0.0 d |
13.3 c 0.0 d |
|||||||
| M06-2X | NC |
NC |
27.9 c 0.0 d |
NC |
143.2 b 22.4 c 0.0 d |
NC |
NC |
NC |
NC |
148.4 b 0.0 d |
NC |
NC |
152.0 b 0.0 d |
NC |
151.7 b 0.0 d |
||||||
| PBEPBE | 98.6 b 14.1 c 0.0 d |
158.3 b 3.3 c 0.0 d |
158.3 b 3.3 c 0.0 d |
155.3 b -0.1 c 0.0 d |
131.3 b -3.0 c 0.0 d |
130.4 b -2.2 c 0.0 d |
132.6 b 0.7 c 0.0 d |
140.0 b 0.6 c 0.0 d |
139.4 b 2.5 c 0.0 d |
135.3 b -1.7 c 0.0 d |
NC |
128.1 b 0.8 c 0.0 d |
140.9 b 2.3 c 0.0 d |
129.9 b 2.5 c 0.0 d |
140.1 b 2.8 c 0.0 d |
2.3 c 0.0 d |
|||||
| PBEPBEultrafine | 131.3 b -3.0 c 0.0 d |
NC |
NC |
NC |
NC |
||||||||||||||||
| PBE1PBE | NC |
NC |
NC |
NC |
130.0 b 8.7 c 0.0 d |
NC |
NC |
NC |
NC |
NC |
NC |
NC |
NC |
NC |
NC |
||||||
| HSEh1PBE | NC |
166.8 b 12.8 c 0.0 d |
NC |
NC |
133.1 b 7.9 c 0.0 d |
NC |
10.9 c 0.0 d |
NC |
NC |
NC |
NC |
NC |
142.1 b 12.4 c 0.0 d |
NC |
NC |
||||||
| TPSSh | 4.5 c 0.0 d |
7.6 c 0.0 d |
5.2 c 0.0 d |
302.9 b 8.7 c 0.0 d |
|||||||||||||||||
| wB97X-D | 23.8 c 0.0 d |
18.5 c 0.0 d |
21.4 c 0.0 d |
23.8 c 0.0 d |
23.2 c 0.0 d |
21.4 c 0.0 d |
23.3 c 0.0 d |
23.5 c 0.0 d |
|||||||||||||
| B97D3 | 333.3 b 6.6 c 0.0 d |
306.3 b 0.9 c 0.0 d |
307.1 b 4.4 c 0.0 d |
313.4 b 6.0 c 0.0 d |
315.2 b 5.8 c 0.0 d |
317.8 b 5.8 c 0.0 d |
316.4 b 5.5 c 0.0 d |
5.8 c 0.0 d |
|||||||||||||
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | cc-pVQZ | aug-cc-pVDZ | aug-cc-pVTZ | aug-cc-pVQZ | cc-pV(T+d)Z | daug-cc-pVTZ | ||
| Moller Plesset perturbation | MP2 | 176.0 b 113.6 c 0.0 d |
218.0 b 62.3 c 0.0 d |
218.0 b 62.3 c 0.0 d |
b c |
49.9 c 0.0 d |
152.1 b 48.8 c 0.0 d |
49.9 c 0.0 d |
153.5 b 50.0 c 0.0 d |
152.7 b 45.1 c 0.0 d |
c |
147.4 b 47.9 c 0.0 d |
153.5 b 46.7 c 0.0 d |
NC |
148.8 b 48.4 c 0.0 d |
152.3 b 0.0 d |
NC |
46.7 c 0.0 d |
|||
| MP2=FULL | NC |
218.0 b 62.1 c 0.0 d |
218.0 b 62.1 c 0.0 d |
207.4 b 62.6 c 0.0 d |
155.5 b 49.1 c 0.0 d |
152.8 b 48.2 c 0.0 d |
153.8 b 49.3 c 0.0 d |
155.4 b 49.4 c 0.0 d |
153.5 b 49.7 c 0.0 d |
152.3 b 0.0 d |
148.1 b 47.5 c 0.0 d |
154.4 b 45.1 c 0.0 d |
NC |
NC |
153.7 b 0.0 d |
NC |
45.1 c 0.0 d |
||||
| MP3 | 141.3 b 37.0 c 0.0 d |
||||||||||||||||||||
| MP3=FULL | 36.3 c 0.0 d |
37.4 c 0.0 d |
|||||||||||||||||||
| MP4 | NC NC |
NC NC |
NC |
NC |
NC |
NC |
|||||||||||||||
| MP4=FULL | NC |
NC |
NC |
NC |
NC |
||||||||||||||||
| B2PLYP | 23.8 c 0.0 d |
0.0 d |
26.0 c 0.0 d |
0.0 d |
|||||||||||||||||
| Configuration interaction | CID | NC |
204.2 b 45.8 c 0.0 d |
191.6 b 44.2 c 0.0 d |
151.5 b 41.2 c 0.0 d |
154.6 b 42.3 c 0.0 d |
|||||||||||||||
| CISD | NC |
204.4 b 45.0 c 0.0 d |
192.2 b 43.4 c 0.0 d |
152.1 b 40.8 c 0.0 d |
154.9 b 42.0 c 0.0 d |
||||||||||||||||
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | cc-pVQZ | aug-cc-pVDZ | aug-cc-pVTZ | aug-cc-pVQZ | cc-pV(T+d)Z | daug-cc-pVTZ | ||
| Quadratic configuration interaction | QCISD | 193.1 b 41.2 c 0.0 d |
193.1 b 41.2 c 0.0 d |
182.0 b 39.8 c 0.0 d |
143.5 b 36.8 c 0.0 d |
140.9 b 36.2 c 0.0 d |
NC |
145.4 b 38.1 c 0.0 d |
NC |
143.4 b 0.0 d |
136.5 b 35.7 c 0.0 d |
145.6 b 0.0 d |
NC |
144.4 b 0.0 d |
|||||||
| QCISD(T) | NC |
NC |
NC |
NC |
NC |
||||||||||||||||
| Coupled Cluster | CCD | 193.3 b 43.7 c 0.0 d |
193.3 b 43.7 c 0.0 d |
181.4 b 0.0 d |
142.4 b 38.8 c 0.0 d |
139.7 b 38.0 c 0.0 d |
NC |
144.8 b 40.0 c 0.0 d |
NC |
NC |
136.1 b 37.1 c 0.0 d |
NC |
NC |
NC |
|||||||
| CCSD | 142.6 b 0.0 d |
0.0 d |
NC |
145.0 b 0.0 d |
NC |
b |
|||||||||||||||
| CCSD=FULL | 143.3 b 0.0 d |
0.0 d |
NC |
146.2 b 0.0 d |
NC |
||||||||||||||||
| CCSD(T) | 137.4 b 32.5 c 0.0 d |
NC |
NC |
||||||||||||||||||
| CCSD(T)=FULL | NC |
NC |
NC |
NC |
NC |
||||||||||||||||
| STO-3G | 3-21G | 3-21G* | 6-31G | 6-31G* | 6-31G** | 6-31+G** | 6-311G* | 6-311G** | 6-31G(2df,p) | 6-311+G(3df,2p) | TZVP | cc-pVDZ | cc-pVTZ | cc-pVQZ | aug-cc-pVDZ | aug-cc-pVTZ | aug-cc-pVQZ | cc-pV(T+d)Z | daug-cc-pVTZ |
| CEP-31G | CEP-31G* | CEP-121G | CEP-121G* | LANL2DZ | SDD | cc-pVTZ-PP | aug-cc-pVTZ-PP | Def2TZVPP | ||
|---|---|---|---|---|---|---|---|---|---|---|
| hartree fock | HF | 158.5 b 54.7 c 0.0 d |
126.9 b 53.1 c 0.0 d |
193.1 b 49.3 c 0.0 d |
169.4 b 49.5 c 0.0 d |
200.1 b 47.0 c 0.0 d |
200.7 b 47.3 c 0.0 d |
45.9 c 0.0 d |
||
| density functional | B1B95 | 22.9 c 0.0 d |
20.3 c 0.0 d |
|||||||
| B3LYP | 131.9 b 23.3 c 0.0 d |
112.8 b 20.3 c 0.0 d |
158.7 b 16.5 c 0.0 d |
146.2 b 13.6 c 0.0 d |
163.4 b 14.6 c 0.0 d |
163.4 b 14.7 c 0.0 d |
11.9 c 0.0 d |
|||
| PBEPBE | 295.8 b 2.1 c 0.0 d |
|||||||||
| Moller Plesset perturbation | MP2 | 164.1 b 69.8 c 0.0 d |
116.3 b 59.9 c 0.0 d |
66.6 c 0.0 d |
143.9 b 54.3 c 0.0 d |
198.3 b 62.5 c 0.0 d |
198.6 b 62.7 c 0.0 d |
46.3 c 0.0 d |
| 6-311+G(3df,2p) | cc-pVDZ | cc-pVTZ | aug-cc-pVDZ | cc-pV(T+d)Z | ||
|---|---|---|---|---|---|---|
| Moller Plesset perturbation | MP2FC// HF/6-31G* | 42.5 c 0.0 d |
42.4 c 0.0 d |
42.4 c 0.0 d |
||
| MP2FC// B3LYP/6-31G* | 46.9 c 0.0 d |
46.9 c 0.0 d |
46.9 c 0.0 d |
46.9 c 0.0 d |
||
| MP2FC// MP2FC/6-31G* | 48.0 c 0.0 d |
48.0 c 0.0 d |
50.1 c 0.0 d |
48.0 c 0.0 d |
||
| MP4// HF/6-31G* | 33.4 c 0.0 d |
33.2 c 0.0 d |
33.2 c 0.0 d |
|||
| MP4// B3LYP/6-31G* | 36.1 c 0.0 d |
36.8 c 0.0 d |
36.8 c 0.0 d |
|||
| MP4// MP2/6-31G* | 38.0 c 0.0 d |
37.8 c 0.0 d |
37.8 c 0.0 d |
|||
| Coupled Cluster | CCSD// HF/6-31G* | 35.5 c 0.0 d |
35.5 c 0.0 d |
|||
| CCSD(T)// HF/6-31G* | 30.6 c 0.0 d |
30.6 c 0.0 d |
||||
| CCSD// B3LYP/6-31G* | 37.7 c 0.0 d |
|||||
| CCSD(T)// B3LYP/6-31G* | 33.6 c 0.0 d |
|||||
| CCSD(T)//B3LYP/6-31G(2df,p) | 32.7 c 0.0 d |
32.7 c 0.0 d |
||||
| CCSD// MP2FC/6-31G* | 37.7 c 0.0 d |
39.3 c 0.0 d |
||||
| CCSD(T)// MP2FC/6-31G* | 34.0 c 0.0 d |
35.8 c 0.0 d |
For descriptions of the methods (AM1, HF, MP2, ...) and basis sets (3-21G, 3-21G*, 6-31G, ...) see the glossary in section I.C. Predefined means the basis set used is determined by the method.
gaw refers to the group additivity method implemeted in the NIST Chemistry Webbook.
See section III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.