Compare Experimental Geometries - experimental data
| Species |
Name |
| CF2Cl2 |
difluorodichloromethane |
The following table lists the experimentally determined internal coordinates.
Coordinate descriptions starting with "r" are bond lengths,
starting with "a" are bond angles, and starting with "d" are dihedral angles.
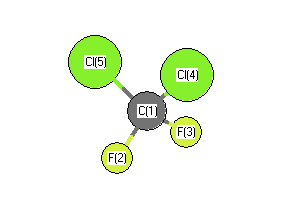
Atom numbers are the numbers on the picture of the molecule below to indicate
which atoms are invloved in the bond or angle.
Units: bond lengths are in Å, angles are in degrees.
| description |
Value |
Reference |
Comment |
Atom number |
| rCCl |
1.744 |
1977tak/mat:636 |
|
1 |
4 |
| |
| rCF |
1.345 |
1977tak/mat:636 |
|
1 |
2 |
| |
| aClCCl |
112.6 |
1977tak/mat:636 |
|
4 |
1 |
5 |
|
| aFCF |
106.2 |
1977tak/mat:636 |
|
2 |
1 |
3 |
|
| aFCCl |
109.5 |
1977tak/mat:636 |
by symmetry |
2 |
1 |
4 |
|
References
| squib | reference |
|---|
| 1977tak/mat:636
| H Takeom C Matsumura "MICROWAVE-SPECTRUM OF DICHLORODIFLUOROMETHANE" Bull. Chem. Soc. Japan 50(3) 636-640, 1977 |
