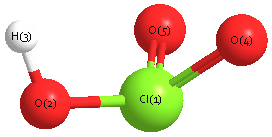Jump to
S1C2
Energy calculated at QCISD(T)/6-31G*
| | hartrees |
|---|
| Energy at 0K | -685.057060 |
| Energy at 298.15K | |
| HF Energy | -684.289861 |
| Nuclear repulsion energy | 188.619924 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at QCISD(T)/6-31G*
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3591 |
3445 |
|
|
|
|
| 2 |
A' |
1211 |
1162 |
|
|
|
|
| 3 |
A' |
964 |
925 |
|
|
|
|
| 4 |
A' |
581 |
557 |
|
|
|
|
| 5 |
A' |
474 |
455 |
|
|
|
|
| 6 |
A' |
367 |
352 |
|
|
|
|
| 7 |
A" |
1120 |
1075 |
|
|
|
|
| 8 |
A" |
366 |
351 |
|
|
|
|
| 9 |
A" |
85i |
82i |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4294.8 cm
-1
Scaled (by 0.9593) Zero Point Vibrational Energy (zpe) 4120.0 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at QCISD(T)/6-31G*
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
0.363 |
0.134 |
0.000 |
| O2 |
-0.207 |
-1.532 |
0.000 |
| H3 |
-1.190 |
-1.430 |
0.000 |
| O4 |
-0.207 |
0.713 |
1.253 |
| O5 |
-0.207 |
0.713 |
-1.253 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.7613 | 2.2043 | 1.4936 | 1.4936 |
O2 | 1.7613 | | 0.9877 | 2.5715 | 2.5715 | H3 | 2.2043 | 0.9877 | | 2.6703 | 2.6703 | O4 | 1.4936 | 2.5715 | 2.6703 | | 2.5060 | O5 | 1.4936 | 2.5715 | 2.6703 | 2.5060 | |
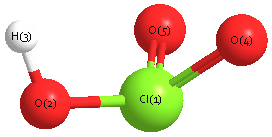 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
34.290 |
|
O2 |
Cl1 |
O3 |
25.891 |
| O2 |
Cl1 |
O4 |
104.078 |
|
O3 |
Cl1 |
O4 |
90.356 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at QCISD(T)/6-31G*
| | hartrees |
|---|
| Energy at 0K | -685.057108 |
| Energy at 298.15K | |
| HF Energy | -684.289845 |
| Nuclear repulsion energy | 188.701234 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at QCISD(T)/6-31G*
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
3592 |
3445 |
|
|
|
|
| 2 |
A |
1215 |
1165 |
|
|
|
|
| 3 |
A |
1118 |
1073 |
|
|
|
|
| 4 |
A |
961 |
922 |
|
|
|
|
| 5 |
A |
584 |
561 |
|
|
|
|
| 6 |
A |
477 |
457 |
|
|
|
|
| 7 |
A |
376 |
361 |
|
|
|
|
| 8 |
A |
340 |
326 |
|
|
|
|
| 9 |
A |
95 |
91 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4378.9 cm
-1
Scaled (by 0.9593) Zero Point Vibrational Energy (zpe) 4200.6 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at QCISD(T)/6-31G*
Point Group is C1
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
-0.174 |
0.020 |
-0.346 |
| O2 |
1.491 |
-0.395 |
0.050 |
| H3 |
1.596 |
-0.008 |
0.952 |
| O4 |
-0.315 |
1.383 |
0.256 |
| O5 |
-1.005 |
-1.029 |
0.310 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.7610 | 2.1957 | 1.4975 | 1.4901 |
O2 | 1.7610 | | 0.9876 | 2.5431 | 2.5879 | H3 | 2.1957 | 0.9876 | | 2.4642 | 2.8673 | O4 | 1.4975 | 2.5431 | 2.4642 | | 2.5097 | O5 | 1.4901 | 2.5879 | 2.8673 | 2.5097 | |
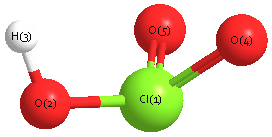 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
33.757 |
|
O2 |
Cl1 |
O3 |
26.063 |
| O2 |
Cl1 |
O4 |
102.299 |
|
O3 |
Cl1 |
O4 |
81.332 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability