Jump to
S1C2
Energy calculated at QCISD(T)/cc-pVDZ
| | hartrees |
|---|
| Energy at 0K | -1194.211758 |
| Energy at 298.15K | -1194.214051 |
| HF Energy | -1193.755575 |
| Nuclear repulsion energy | 192.209366 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at QCISD(T)/cc-pVDZ
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
2678 |
2552 |
|
|
|
|
| 2 |
A |
872 |
831 |
|
|
|
|
| 3 |
A |
475 |
453 |
|
|
|
|
| 4 |
A |
302 |
288 |
|
|
|
|
| 5 |
A |
199 |
190 |
|
|
|
|
| 6 |
B |
2677 |
2551 |
|
|
|
|
| 7 |
B |
863 |
823 |
|
|
|
|
| 8 |
B |
469 |
447 |
|
|
|
|
| 9 |
B |
328 |
313 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4431.5 cm
-1
Scaled (by 0.9531) Zero Point Vibrational Energy (zpe) 4223.7 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
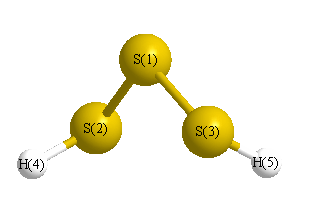
Geometric Data calculated at QCISD(T)/cc-pVDZ
Point Group is C2
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| S1 |
0.000 |
0.000 |
0.869 |
| S2 |
0.000 |
1.686 |
-0.398 |
| S3 |
0.000 |
-1.686 |
-0.398 |
| H4 |
-1.342 |
1.760 |
-0.588 |
| H5 |
1.342 |
-1.760 |
-0.588 |
Atom - Atom Distances (Å)
| |
S1 |
S2 |
S3 |
H4 |
H5 |
| S1 | | 2.1088 | 2.1088 | 2.6499 | 2.6499 |
S2 | 2.1088 | | 3.3721 | 1.3578 | 3.7034 | S3 | 2.1088 | 3.3721 | | 3.7034 | 1.3578 | H4 | 2.6499 | 1.3578 | 3.7034 | | 4.4275 | H5 | 2.6499 | 3.7034 | 1.3578 | 4.4275 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| S1 |
S2 |
H4 |
97.341 |
|
S1 |
S3 |
H5 |
97.341 |
| S2 |
S1 |
S3 |
106.174 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at QCISD(T)/cc-pVDZ
| | hartrees |
|---|
| Energy at 0K | -1194.211438 |
| Energy at 298.15K | -1194.213737 |
| HF Energy | -1193.755101 |
| Nuclear repulsion energy | 192.221962 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at QCISD(T)/cc-pVDZ
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
2670 |
2545 |
|
|
|
|
| 2 |
A' |
877 |
836 |
|
|
|
|
| 3 |
A' |
476 |
453 |
|
|
|
|
| 4 |
A' |
328 |
312 |
|
|
|
|
| 5 |
A' |
200 |
191 |
|
|
|
|
| 6 |
A" |
2672 |
2547 |
|
|
|
|
| 7 |
A" |
865 |
824 |
|
|
|
|
| 8 |
A" |
469 |
447 |
|
|
|
|
| 9 |
A" |
304 |
290 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4430.2 cm
-1
Scaled (by 0.9531) Zero Point Vibrational Energy (zpe) 4222.4 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at QCISD(T)/cc-pVDZ
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| S1 |
-0.054 |
0.866 |
0.000 |
| S2 |
-0.054 |
-0.400 |
1.685 |
| S3 |
-0.054 |
-0.400 |
-1.685 |
| H4 |
1.293 |
-0.524 |
1.807 |
| H5 |
1.293 |
-0.524 |
-1.807 |
Atom - Atom Distances (Å)
| |
S1 |
S2 |
S3 |
H4 |
H5 |
| S1 | | 2.1084 | 2.1084 | 2.6483 | 2.6483 |
S2 | 2.1084 | | 3.3709 | 1.3584 | 3.7451 | S3 | 2.1084 | 3.3709 | | 3.7451 | 1.3584 | H4 | 2.6483 | 1.3584 | 3.7451 | | 3.6134 | H5 | 2.6483 | 3.7451 | 1.3584 | 3.6134 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| S1 |
S2 |
H4 |
97.253 |
|
S1 |
S3 |
H5 |
97.253 |
| S2 |
S1 |
S3 |
106.150 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
