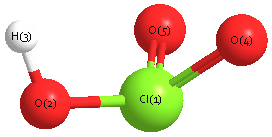Jump to
S1C2
Energy calculated at CCSD/cc-pVTZ
| | hartrees |
|---|
| Energy at 0K | -685.419018 |
| Energy at 298.15K | |
| HF Energy | -684.475547 |
| Nuclear repulsion energy | 195.851764 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCSD/cc-pVTZ
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3755 |
3534 |
93.29 |
|
|
|
| 2 |
A' |
1250 |
1176 |
56.10 |
|
|
|
| 3 |
A' |
1071 |
1008 |
62.76 |
|
|
|
| 4 |
A' |
682 |
642 |
208.26 |
|
|
|
| 5 |
A' |
552 |
520 |
25.35 |
|
|
|
| 6 |
A' |
445 |
418 |
10.95 |
|
|
|
| 7 |
A" |
1223 |
1151 |
248.32 |
|
|
|
| 8 |
A" |
434 |
408 |
36.56 |
|
|
|
| 9 |
A" |
112i |
105i |
62.00 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4649.6 cm
-1
Scaled (by 0.9412) Zero Point Vibrational Energy (zpe) 4376.2 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CCSD/cc-pVTZ
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
0.358 |
0.116 |
0.000 |
| O2 |
-0.205 |
-1.460 |
0.000 |
| H3 |
-1.170 |
-1.353 |
0.000 |
| O4 |
-0.205 |
0.691 |
1.204 |
| O5 |
-0.205 |
0.691 |
-1.204 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.6737 | 2.1203 | 1.4482 | 1.4482 |
O2 | 1.6737 | | 0.9705 | 2.4645 | 2.4645 | H3 | 2.1203 | 0.9705 | | 2.5610 | 2.5610 | O4 | 1.4482 | 2.4645 | 2.5610 | | 2.4079 | O5 | 1.4482 | 2.4645 | 2.5610 | 2.4079 | |
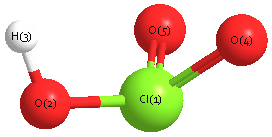 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
34.755 |
|
O2 |
Cl1 |
O3 |
26.442 |
| O2 |
Cl1 |
O4 |
104.034 |
|
O3 |
Cl1 |
O4 |
89.683 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at CCSD/cc-pVTZ
| | hartrees |
|---|
| Energy at 0K | -685.419133 |
| Energy at 298.15K | |
| HF Energy | -684.475227 |
| Nuclear repulsion energy | 195.890331 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCSD/cc-pVTZ
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
3761 |
3540 |
101.59 |
|
|
|
| 2 |
A |
1261 |
1187 |
103.21 |
|
|
|
| 3 |
A |
1218 |
1146 |
197.60 |
|
|
|
| 4 |
A |
1066 |
1003 |
63.90 |
|
|
|
| 5 |
A |
689 |
648 |
203.14 |
|
|
|
| 6 |
A |
556 |
524 |
28.78 |
|
|
|
| 7 |
A |
449 |
422 |
12.40 |
|
|
|
| 8 |
A |
401 |
377 |
17.69 |
|
|
|
| 9 |
A |
142 |
134 |
79.26 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4771.1 cm
-1
Scaled (by 0.9412) Zero Point Vibrational Energy (zpe) 4490.6 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CCSD/cc-pVTZ
Point Group is C1
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
-0.159 |
0.020 |
-0.343 |
| O2 |
1.396 |
-0.457 |
0.063 |
| H3 |
1.563 |
0.041 |
0.878 |
| O4 |
-0.235 |
1.347 |
0.245 |
| O5 |
-1.019 |
-0.936 |
0.311 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.6763 | 2.1113 | 1.4535 | 1.4427 |
O2 | 1.6763 | | 0.9700 | 2.4389 | 2.4746 | H3 | 2.1113 | 0.9700 | | 2.3110 | 2.8185 | O4 | 1.4535 | 2.4389 | 2.3110 | | 2.4144 | O5 | 1.4427 | 2.4746 | 2.8185 | 2.4144 | |
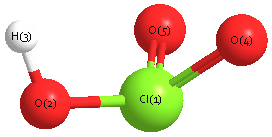 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
34.318 |
|
O2 |
Cl1 |
O3 |
26.643 |
| O2 |
Cl1 |
O4 |
102.154 |
|
O3 |
Cl1 |
O4 |
78.445 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability