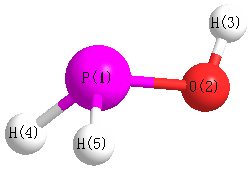Jump to
S1C2
Energy calculated at MP4/cc-pV(T+d)Z
| | hartrees |
|---|
| Energy at 0K | -417.839833 |
| Energy at 298.15K | -417.843969 |
| HF Energy | -417.398489 |
| Nuclear repulsion energy | 61.755781 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4/cc-pV(T+d)Z
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3845 |
3845 |
|
|
|
|
| 2 |
A' |
2364 |
2364 |
|
|
|
|
| 3 |
A' |
1157 |
1157 |
|
|
|
|
| 4 |
A' |
1123 |
1123 |
|
|
|
|
| 5 |
A' |
924 |
924 |
|
|
|
|
| 6 |
A' |
815 |
815 |
|
|
|
|
| 7 |
A" |
2365 |
2365 |
|
|
|
|
| 8 |
A" |
926 |
926 |
|
|
|
|
| 9 |
A" |
423 |
423 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 6970.3 cm
-1
Scaled (by 1) Zero Point Vibrational Energy (zpe) 6970.3 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at MP4/cc-pV(T+d)Z
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| P1 |
-0.107 |
-0.565 |
0.000 |
| O2 |
-0.107 |
1.089 |
0.000 |
| H3 |
0.780 |
1.462 |
0.000 |
| H4 |
0.841 |
-0.848 |
1.022 |
| H5 |
0.841 |
-0.848 |
-1.022 |
Atom - Atom Distances (Å)
| |
P1 |
O2 |
H3 |
H4 |
H5 |
| P1 | | 1.6546 | 2.2124 | 1.4222 | 1.4222 |
O2 | 1.6546 | | 0.9617 | 2.3863 | 2.3863 | H3 | 2.2124 | 0.9617 | | 2.5259 | 2.5259 | H4 | 1.4222 | 2.3863 | 2.5259 | | 2.0436 | H5 | 1.4222 | 2.3863 | 2.5259 | 2.0436 | |
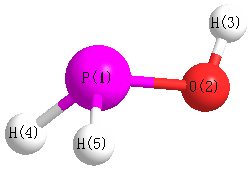 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| P1 |
O2 |
H3 |
112.774 |
|
O2 |
P1 |
H4 |
101.448 |
| O2 |
P1 |
H5 |
101.448 |
|
H4 |
P1 |
H5 |
91.851 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at MP4/cc-pV(T+d)Z
| | hartrees |
|---|
| Energy at 0K | -417.839850 |
| Energy at 298.15K | -417.843827 |
| HF Energy | -417.397758 |
| Nuclear repulsion energy | 61.661755 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4/cc-pV(T+d)Z
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3867 |
3867 |
|
|
|
|
| 2 |
A' |
2402 |
2402 |
|
|
|
|
| 3 |
A' |
1167 |
1167 |
|
|
|
|
| 4 |
A' |
1158 |
1158 |
|
|
|
|
| 5 |
A' |
922 |
922 |
|
|
|
|
| 6 |
A' |
808 |
808 |
|
|
|
|
| 7 |
A" |
2400 |
2400 |
|
|
|
|
| 8 |
A" |
940 |
940 |
|
|
|
|
| 9 |
A" |
273 |
273 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 6968.0 cm
-1
Scaled (by 1) Zero Point Vibrational Energy (zpe) 6968.0 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at MP4/cc-pV(T+d)Z
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| P1 |
0.038 |
-0.571 |
0.000 |
| O2 |
0.038 |
1.094 |
0.000 |
| H3 |
0.954 |
1.386 |
0.000 |
| H4 |
-0.918 |
-0.786 |
1.023 |
| H5 |
-0.918 |
-0.786 |
-1.023 |
Atom - Atom Distances (Å)
| |
P1 |
O2 |
H3 |
H4 |
H5 |
| P1 | | 1.6643 | 2.1601 | 1.4165 | 1.4165 |
O2 | 1.6643 | | 0.9609 | 2.3436 | 2.3436 | H3 | 2.1601 | 0.9609 | | 3.0439 | 3.0439 | H4 | 1.4165 | 2.3436 | 3.0439 | | 2.0452 | H5 | 1.4165 | 2.3436 | 3.0439 | 2.0452 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| P1 |
O2 |
H3 |
107.710 |
|
O2 |
P1 |
H4 |
98.737 |
| O2 |
P1 |
H5 |
98.737 |
|
H4 |
P1 |
H5 |
92.424 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability