All results from a given calculation for C3H3NS (Thiazole)
using model chemistry: MP4=FULL/6-311+G(3df,2p)
19 10 17 12 22
States and conformations
| State |
Conformation |
minimum conformation |
conformer description |
state description |
| 1 |
1 |
yes |
CS |
1A' |
Energy calculated at MP4=FULL/6-311+G(3df,2p)
| | hartrees |
|---|
| Energy at 0K | -568.557023 |
| Energy at 298.15K | |
| HF Energy | -567.376192 |
| Nuclear repulsion energy | 205.312580 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4=FULL/6-311+G(3df,2p)
Geometric Data calculated at MP4=FULL/6-311+G(3df,2p)
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| S1 |
0.000 |
1.179 |
0.000 |
| C2 |
-1.198 |
-0.058 |
0.000 |
| C3 |
1.217 |
-0.023 |
0.000 |
| N4 |
-0.734 |
-1.285 |
0.000 |
| C5 |
0.637 |
-1.267 |
0.000 |
| H6 |
-2.251 |
0.186 |
0.000 |
| H7 |
2.265 |
0.233 |
0.000 |
| H8 |
1.179 |
-2.202 |
0.000 |
Atom - Atom Distances (Å)
| |
S1 |
C2 |
C3 |
N4 |
C5 |
H6 |
H7 |
H8 |
| S1 | | 1.7222 | 1.7107 | 2.5713 | 2.5273 | 2.4598 | 2.4545 | 3.5806 |
C2 | 1.7222 | | 2.4153 | 1.3117 | 2.1971 | 1.0808 | 3.4750 | 3.2005 | C3 | 1.7107 | 2.4153 | | 2.3239 | 1.3723 | 3.4742 | 1.0785 | 2.1794 | N4 | 2.5713 | 1.3117 | 2.3239 | | 1.3712 | 2.1133 | 3.3613 | 2.1209 | C5 | 2.5273 | 2.1971 | 1.3723 | 1.3712 | | 3.2327 | 2.2133 | 1.0808 | H6 | 2.4598 | 1.0808 | 3.4742 | 2.1133 | 3.2327 | | 4.5158 | 4.1790 | H7 | 2.4545 | 3.4750 | 1.0785 | 3.3613 | 2.2133 | 4.5158 | | 2.6664 | H8 | 3.5806 | 3.2005 | 2.1794 | 2.1209 | 1.0808 | 4.1790 | 2.6664 | |
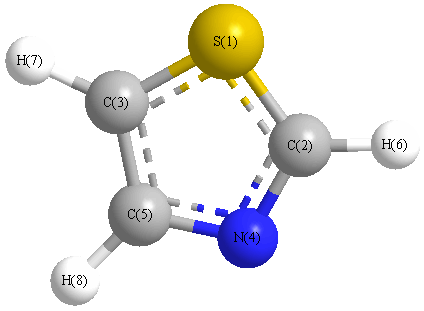 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| S1 |
C2 |
N4 |
115.217 |
|
S1 |
C2 |
H6 |
120.987 |
| S1 |
C3 |
C5 |
109.637 |
|
S1 |
C3 |
H7 |
121.626 |
| C2 |
S1 |
C3 |
89.430 |
|
C2 |
N4 |
C5 |
109.934 |
| C3 |
C5 |
N4 |
115.783 |
|
C3 |
C5 |
H8 |
124.933 |
| N4 |
C2 |
H6 |
123.796 |
|
N4 |
C5 |
H8 |
119.284 |
| C5 |
C3 |
H7 |
128.738 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
