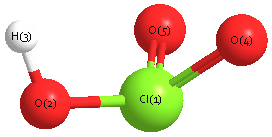Jump to
S1C2
Energy calculated at CCD/TZVP
| | hartrees |
|---|
| Energy at 0K | -685.181511 |
| Energy at 298.15K | -685.184226 |
| HF Energy | -684.406880 |
| Nuclear repulsion energy | 192.751674 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCD/TZVP
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3775 |
3579 |
82.53 |
|
|
|
| 2 |
A' |
1222 |
1158 |
57.60 |
|
|
|
| 3 |
A' |
1047 |
993 |
82.96 |
|
|
|
| 4 |
A' |
649 |
615 |
226.16 |
|
|
|
| 5 |
A' |
533 |
505 |
38.40 |
|
|
|
| 6 |
A' |
423 |
401 |
8.96 |
|
|
|
| 7 |
A" |
1176 |
1115 |
312.48 |
|
|
|
| 8 |
A" |
432 |
409 |
60.51 |
|
|
|
| 9 |
A" |
138 |
130 |
52.84 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4696.8 cm
-1
Scaled (by 0.948) Zero Point Vibrational Energy (zpe) 4452.6 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CCD/TZVP
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
0.367 |
0.120 |
0.000 |
| O2 |
-0.211 |
-1.487 |
0.000 |
| H3 |
-1.176 |
-1.375 |
0.000 |
| O4 |
-0.211 |
0.702 |
1.220 |
| O5 |
-0.211 |
0.702 |
-1.220 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.7079 | 2.1486 | 1.4702 | 1.4702 |
O2 | 1.7079 | | 0.9717 | 2.5059 | 2.5059 | H3 | 2.1486 | 0.9717 | | 2.5947 | 2.5947 | O4 | 1.4702 | 2.5059 | 2.5947 | | 2.4406 | O5 | 1.4702 | 2.5059 | 2.5947 | 2.4406 | |
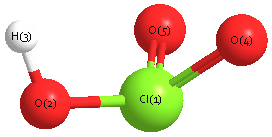 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
34.729 |
|
O2 |
Cl1 |
O3 |
26.127 |
| O2 |
Cl1 |
O4 |
103.834 |
|
O3 |
Cl1 |
O4 |
89.586 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at CCD/TZVP
| | hartrees |
|---|
| Energy at 0K | -685.181511 |
| Energy at 298.15K | |
| HF Energy | -684.406904 |
| Nuclear repulsion energy | 192.773912 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCD/TZVP
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
3776 |
3579 |
82.48 |
|
|
|
| 2 |
A |
1222 |
1159 |
57.64 |
|
|
|
| 3 |
A |
1177 |
1115 |
312.59 |
|
|
|
| 4 |
A |
1048 |
993 |
82.99 |
|
|
|
| 5 |
A |
649 |
615 |
226.23 |
|
|
|
| 6 |
A |
533 |
505 |
38.42 |
|
|
|
| 7 |
A |
432 |
410 |
60.65 |
|
|
|
| 8 |
A |
423 |
401 |
9.01 |
|
|
|
| 9 |
A |
137 |
130 |
52.71 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4698.0 cm
-1
Scaled (by 0.948) Zero Point Vibrational Energy (zpe) 4453.8 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CCD/TZVP
Point Group is C1
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
-0.159 |
-0.000 |
-0.352 |
| O2 |
1.501 |
0.001 |
0.050 |
| H3 |
1.493 |
0.001 |
1.022 |
| O4 |
-0.676 |
1.220 |
0.285 |
| O5 |
-0.674 |
-1.220 |
0.285 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.7077 | 2.1481 | 1.4700 | 1.4701 |
O2 | 1.7077 | | 0.9717 | 2.5058 | 2.5056 | H3 | 2.1481 | 0.9717 | | 2.5943 | 2.5943 | O4 | 1.4700 | 2.5058 | 2.5943 | | 2.4403 | O5 | 1.4701 | 2.5056 | 2.5943 | 2.4403 | |
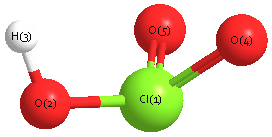 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
34.728 |
|
O2 |
Cl1 |
O3 |
26.136 |
| O2 |
Cl1 |
O4 |
103.848 |
|
O3 |
Cl1 |
O4 |
89.590 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability