All results from a given calculation for CF3CHFCl (1,1,1,2-tetrafluorochloroethane)
using model chemistry: MP3/6-31+G**
19 10 17 12 22
States and conformations
| State |
Conformation |
minimum conformation |
conformer description |
state description |
| 1 |
1 |
yes |
C1 |
1A |
Energy calculated at MP3/6-31+G**
| | hartrees |
|---|
| Energy at 0K | -934.633903 |
| Energy at 298.15K | |
| HF Energy | -933.551442 |
| Nuclear repulsion energy | 421.356370 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP3/6-31+G**
Geometric Data calculated at MP3/6-31+G**
Point Group is C1
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| C1 |
0.442 |
0.478 |
-0.474 |
| C2 |
-0.855 |
-0.178 |
-0.000 |
| H3 |
0.433 |
0.537 |
-1.559 |
| F4 |
0.493 |
1.723 |
0.067 |
| Cl5 |
1.843 |
-0.471 |
0.021 |
| F6 |
-0.889 |
-0.280 |
1.327 |
| F7 |
-1.881 |
0.581 |
-0.403 |
| F8 |
-0.979 |
-1.395 |
-0.540 |
Atom - Atom Distances (Å)
| |
C1 |
C2 |
H3 |
F4 |
Cl5 |
F6 |
F7 |
F8 |
| C1 | | 1.5285 | 1.0868 | 1.3585 | 1.7634 | 2.3639 | 2.3264 | 2.3520 |
C2 | 1.5285 | | 2.1447 | 2.3316 | 2.7139 | 1.3313 | 1.3391 | 1.3371 | H3 | 1.0868 | 2.1447 | | 2.0137 | 2.3460 | 3.2776 | 2.5868 | 2.6009 | F4 | 1.3585 | 2.3316 | 2.0137 | | 2.5764 | 2.7402 | 2.6763 | 3.5013 | Cl5 | 1.7634 | 2.7139 | 2.3460 | 2.5764 | | 3.0341 | 3.8934 | 3.0224 | F6 | 2.3639 | 1.3313 | 3.2776 | 2.7402 | 3.0341 | | 2.1725 | 2.1766 | F7 | 2.3264 | 1.3391 | 2.5868 | 2.6763 | 3.8934 | 2.1725 | | 2.1769 | F8 | 2.3520 | 1.3371 | 2.6009 | 3.5013 | 3.0224 | 2.1766 | 2.1769 | |
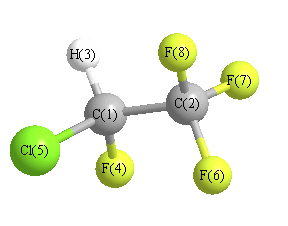 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C2 |
F6 |
111.319 |
|
C1 |
C2 |
F7 |
108.259 |
| C1 |
C2 |
F8 |
110.145 |
|
C2 |
C1 |
H3 |
109.011 |
| C2 |
C1 |
F4 |
107.583 |
|
C2 |
C1 |
Cl5 |
110.856 |
| H3 |
C1 |
F4 |
110.377 |
|
H3 |
C1 |
Cl5 |
108.448 |
| F4 |
C1 |
Cl5 |
110.558 |
|
F6 |
C2 |
F7 |
108.886 |
| F6 |
C2 |
F8 |
109.313 |
|
F7 |
C2 |
F8 |
108.870 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
