All results from a given calculation for C3H3NS (Thiazole)
using model chemistry: CCSD(T)=FULL/6-311+G(3df,2p)
19 10 17 12 22
States and conformations
| State |
Conformation |
minimum conformation |
conformer description |
state description |
| 1 |
1 |
yes |
CS |
1A' |
Energy calculated at CCSD(T)=FULL/6-311+G(3df,2p)
| | hartrees |
|---|
| Energy at 0K | -568.548542 |
| Energy at 298.15K | |
| HF Energy | -567.376988 |
| Nuclear repulsion energy | 205.444834 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCSD(T)=FULL/6-311+G(3df,2p)
Geometric Data calculated at CCSD(T)=FULL/6-311+G(3df,2p)
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| S1 |
0.000 |
1.179 |
0.000 |
| C2 |
-1.196 |
-0.060 |
0.000 |
| C3 |
1.218 |
-0.027 |
0.000 |
| N4 |
-0.736 |
-1.281 |
0.000 |
| C5 |
0.638 |
-1.265 |
0.000 |
| H6 |
-2.248 |
0.183 |
0.000 |
| H7 |
2.266 |
0.225 |
0.000 |
| H8 |
1.176 |
-2.201 |
0.000 |
Atom - Atom Distances (Å)
| |
S1 |
C2 |
C3 |
N4 |
C5 |
H6 |
H7 |
H8 |
| S1 | | 1.7225 | 1.7137 | 2.5685 | 2.5259 | 2.4592 | 2.4584 | 3.5790 |
C2 | 1.7225 | | 2.4138 | 1.3049 | 2.1942 | 1.0800 | 3.4732 | 3.1951 | C3 | 1.7137 | 2.4138 | | 2.3220 | 1.3669 | 3.4722 | 1.0776 | 2.1747 | N4 | 2.5685 | 1.3049 | 2.3220 | | 1.3744 | 2.1049 | 3.3583 | 2.1218 | C5 | 2.5259 | 2.1942 | 1.3669 | 1.3744 | | 3.2290 | 2.2060 | 1.0798 | H6 | 2.4592 | 1.0800 | 3.4722 | 2.1049 | 3.2290 | | 4.5140 | 4.1723 | H7 | 2.4584 | 3.4732 | 1.0776 | 3.3583 | 2.2060 | 4.5140 | | 2.6590 | H8 | 3.5790 | 3.1951 | 2.1747 | 2.1218 | 1.0798 | 4.1723 | 2.6590 | |
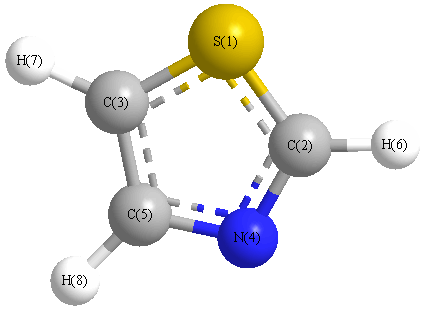 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| S1 |
C2 |
N4 |
115.391 |
|
S1 |
C2 |
H6 |
120.966 |
| S1 |
C3 |
C5 |
109.642 |
|
S1 |
C3 |
H7 |
121.788 |
| C2 |
S1 |
C3 |
89.250 |
|
C2 |
N4 |
C5 |
109.934 |
| C3 |
C5 |
N4 |
115.783 |
|
C3 |
C5 |
H8 |
125.040 |
| N4 |
C2 |
H6 |
123.643 |
|
N4 |
C5 |
H8 |
119.177 |
| C5 |
C3 |
H7 |
128.570 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
