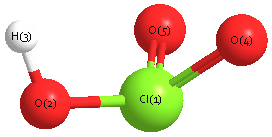Jump to
S1C2
Energy calculated at CCSD(T)=FULL/TZVP
| | hartrees |
|---|
| Energy at 0K | -685.315529 |
| Energy at 298.15K | -685.318009 |
| HF Energy | -684.398111 |
| Nuclear repulsion energy | 188.276150 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCSD(T)=FULL/TZVP
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3710 |
3546 |
|
|
|
|
| 2 |
A' |
1142 |
1092 |
|
|
|
|
| 3 |
A' |
953 |
911 |
|
|
|
|
| 4 |
A' |
557 |
533 |
|
|
|
|
| 5 |
A' |
476 |
455 |
|
|
|
|
| 6 |
A' |
362 |
346 |
|
|
|
|
| 7 |
A" |
1085 |
1037 |
|
|
|
|
| 8 |
A" |
375 |
359 |
|
|
|
|
| 9 |
A" |
146 |
139 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4402.3 cm
-1
Scaled (by 0.956) Zero Point Vibrational Energy (zpe) 4208.6 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CCSD(T)=FULL/TZVP
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
0.372 |
0.132 |
0.000 |
| O2 |
-0.214 |
-1.544 |
0.000 |
| H3 |
-1.181 |
-1.409 |
0.000 |
| O4 |
-0.214 |
0.720 |
1.244 |
| O5 |
-0.214 |
0.720 |
-1.244 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.7747 | 2.1872 | 1.4956 | 1.4956 |
O2 | 1.7747 | | 0.9758 | 2.5827 | 2.5827 | H3 | 2.1872 | 0.9758 | | 2.6484 | 2.6484 | O4 | 1.4956 | 2.5827 | 2.6484 | | 2.4881 | O5 | 1.4956 | 2.5827 | 2.6484 | 2.4881 | |
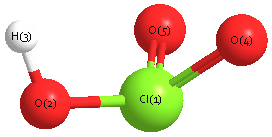 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
34.186 |
|
O2 |
Cl1 |
O3 |
25.937 |
| O2 |
Cl1 |
O4 |
103.998 |
|
O3 |
Cl1 |
O4 |
89.943 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at CCSD(T)=FULL/TZVP
| | hartrees |
|---|
| Energy at 0K | -685.315529 |
| Energy at 298.15K | |
| HF Energy | -684.398125 |
| Nuclear repulsion energy | 188.284233 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCSD(T)=FULL/TZVP
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
3709 |
3546 |
|
|
|
|
| 2 |
A |
1143 |
1093 |
|
|
|
|
| 3 |
A |
1087 |
1039 |
|
|
|
|
| 4 |
A |
958 |
916 |
|
|
|
|
| 5 |
A |
558 |
534 |
|
|
|
|
| 6 |
A |
487 |
466 |
|
|
|
|
| 7 |
A |
376 |
360 |
|
|
|
|
| 8 |
A |
365 |
349 |
|
|
|
|
| 9 |
A |
130 |
124 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4406.5 cm
-1
Scaled (by 0.956) Zero Point Vibrational Energy (zpe) 4212.6 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CCSD(T)=FULL/TZVP
Point Group is C1
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
-0.170 |
-0.000 |
-0.356 |
| O2 |
1.557 |
0.006 |
0.049 |
| H3 |
1.526 |
0.000 |
1.025 |
| O4 |
-0.698 |
1.241 |
0.290 |
| O5 |
-0.688 |
-1.247 |
0.289 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.7746 | 2.1874 | 1.4955 | 1.4957 |
O2 | 1.7746 | | 0.9758 | 2.5828 | 2.5819 | H3 | 2.1874 | 0.9758 | | 2.6509 | 2.6453 | O4 | 1.4955 | 2.5828 | 2.6509 | | 2.4878 | O5 | 1.4957 | 2.5819 | 2.6453 | 2.4878 | |
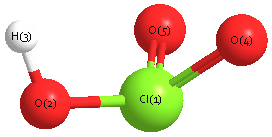 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
34.209 |
|
O2 |
Cl1 |
O3 |
25.935 |
| O2 |
Cl1 |
O4 |
104.010 |
|
O3 |
Cl1 |
O4 |
90.054 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability