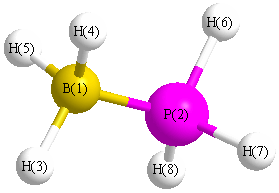All results from a given calculation for BH3PH3 (borane phosphine)
using model chemistry: QCISD(T)=FULL/cc-pVQZ
19 10 17 12 22
States and conformations
| State |
Conformation |
minimum conformation |
conformer description |
state description |
| 1 |
1 |
yes |
C3V |
1A1 |
Energy calculated at QCISD(T)=FULL/cc-pVQZ
| | hartrees |
|---|
| Energy at 0K | -369.374877 |
| Energy at 298.15K | |
| HF Energy | -368.912686 |
| Nuclear repulsion energy | 59.499846 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at QCISD(T)=FULL/cc-pVQZ
Geometric Data calculated at QCISD(T)=FULL/cc-pVQZ
Point Group is C3v
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| B1 |
0.000 |
0.000 |
-1.377 |
| P2 |
0.000 |
0.000 |
0.551 |
| H3 |
0.000 |
-1.170 |
-1.659 |
| H4 |
-1.013 |
0.585 |
-1.659 |
| H5 |
1.013 |
0.585 |
-1.659 |
| H6 |
0.000 |
1.237 |
1.201 |
| H7 |
-1.071 |
-0.618 |
1.201 |
| H8 |
1.071 |
-0.618 |
1.201 |
Atom - Atom Distances (Å)
| |
B1 |
P2 |
H3 |
H4 |
H5 |
H6 |
H7 |
H8 |
| B1 | | 1.9277 | 1.2032 | 1.2032 | 1.2032 | 2.8597 | 2.8597 | 2.8597 |
P2 | 1.9277 | | 2.4996 | 2.4996 | 2.4996 | 1.3975 | 1.3975 | 1.3975 | H3 | 1.2032 | 2.4996 | | 2.0262 | 2.0262 | 3.7376 | 3.1031 | 3.1031 | H4 | 1.2032 | 2.4996 | 2.0262 | | 2.0262 | 3.1031 | 3.1031 | 3.7376 | H5 | 1.2032 | 2.4996 | 2.0262 | 2.0262 | | 3.1031 | 3.7376 | 3.1031 | H6 | 2.8597 | 1.3975 | 3.7376 | 3.1031 | 3.1031 | | 2.1422 | 2.1422 | H7 | 2.8597 | 1.3975 | 3.1031 | 3.1031 | 3.7376 | 2.1422 | | 2.1422 | H8 | 2.8597 | 1.3975 | 3.1031 | 3.7376 | 3.1031 | 2.1422 | 2.1422 | |
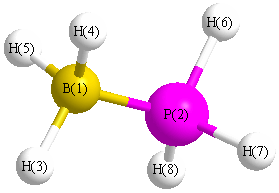 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| B1 |
P2 |
H6 |
117.749 |
|
B1 |
P2 |
H7 |
117.749 |
| B1 |
P2 |
H8 |
117.749 |
|
P2 |
B1 |
H3 |
103.524 |
| P2 |
B1 |
H4 |
103.524 |
|
P2 |
B1 |
H5 |
103.524 |
| H3 |
B1 |
H4 |
114.707 |
|
H3 |
B1 |
H5 |
114.707 |
| H4 |
B1 |
H5 |
114.707 |
|
H6 |
P2 |
H7 |
100.068 |
| H6 |
P2 |
H8 |
100.068 |
|
H7 |
P2 |
H8 |
100.068 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability