Geometric Data
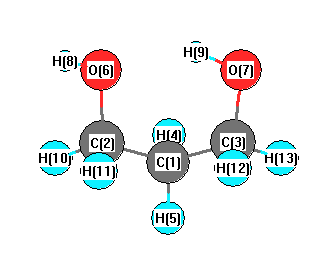
Point Group C1
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| C1 |
-0.4040 |
1.1647 |
0.0128 |
| C2 |
-1.3687 |
-0.0019 |
-0.0138 |
| C3 |
1.0393 |
0.7076 |
-0.0018 |
| H4 |
-0.5895 |
1.7783 |
0.9555 |
| H5 |
-0.5937 |
1.8240 |
-0.8977 |
| O6 |
-0.6128 |
-1.1917 |
-0.0448 |
| O7 |
1.1479 |
-0.6978 |
-0.0367 |
| H8 |
0.1470 |
-1.1288 |
0.6626 |
| H9 |
2.0963 |
-0.9444 |
-0.0449 |
| H10 |
-2.0271 |
0.0398 |
0.9159 |
| H11 |
-2.0314 |
0.0855 |
-0.9372 |
| H12 |
1.5614 |
1.0971 |
0.9337 |
| H13 |
1.5572 |
1.1427 |
-0.9194 |
Atom - Atom Distances 
Distances in Å
| |
C1 |
C2 |
C3 |
H4 |
H5 |
O6 |
O7 |
H8 |
H9 |
H10 |
H11 |
H12 |
H13 |
| C1 |
|
1.5140 | 1.5140 | 1.1400 | 1.1400 | 2.3664 | 2.4248 | 2.4466 | 3.2716 | 2.1716 | 2.1716 | 2.1715 | 2.1716 |
| C2 |
1.5140 |
|
2.5103 | 2.1716 | 2.1716 | 1.4100 | 2.6111 | 2.0061 | 3.5910 | 1.1400 | 1.1400 | 3.2697 | 3.2697 |
| C3 |
1.5140 | 2.5103 |
|
2.1716 | 2.1716 | 2.5177 | 1.4100 | 2.1470 | 1.9617 | 3.2697 | 3.2697 | 1.1400 | 1.1400 |
| H4 |
1.1400 | 2.1716 | 2.1716 |
|
1.8537 | 3.1340 | 3.1834 | 3.0132 | 3.9532 | 2.2563 | 2.9201 | 2.2563 | 2.9201 |
| H5 |
1.1400 | 2.1716 | 2.1716 | 1.8537 |
|
3.1340 | 3.1834 | 3.4208 | 3.9532 | 2.9201 | 2.2563 | 2.9201 | 2.2563 |
| O6 |
2.3664 | 1.4100 | 2.5177 | 3.1340 | 3.1340 |
|
1.8287 | 1.0400 | 2.7204 | 2.1071 | 2.1071 | 3.3051 | 3.3051 |
| O7 |
2.4248 | 2.6111 | 1.4100 | 3.1834 | 3.1834 | 1.8287 |
|
1.2948 | 0.9800 | 3.3959 | 3.3959 | 2.0819 | 2.0819 |
| H8 |
2.4466 | 2.0061 | 2.1470 | 3.0132 | 3.4208 | 1.0400 | 1.2948 |
|
2.0819 | 2.4812 | 2.9629 | 2.6512 | 3.1066 |
| H9 |
3.2716 | 3.5910 | 1.9617 | 3.9532 | 3.9532 | 2.7204 | 0.9800 | 2.0819 |
|
4.3468 | 4.3468 | 2.3263 | 2.3263 |
| H10 |
2.1716 | 1.1400 | 3.2697 | 2.2563 | 2.9201 | 2.1071 | 3.3959 | 2.4812 | 4.3468 |
|
1.8537 | 3.7411 | 4.1752 |
| H11 |
2.1716 | 1.1400 | 3.2697 | 2.9201 | 2.2563 | 2.1071 | 3.3959 | 2.9629 | 4.3468 | 1.8537 |
|
4.1752 | 3.7411 |
| H12 |
2.1715 | 3.2697 | 1.1400 | 2.2563 | 2.9201 | 3.3051 | 2.0819 | 2.6512 | 2.3263 | 3.7411 | 4.1752 |
|
1.8537 |
| H13 |
2.1716 | 3.2697 | 1.1400 | 2.9201 | 2.2563 | 3.3051 | 2.0819 | 3.1066 | 2.3263 | 4.1752 | 3.7411 | 1.8537 |
|
Calculated geometries
for C
3H
8O
2 (1,3-Propanediol).
Experimental Bond Angles (degrees) from cartesians 
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C2 |
O6 |
108.000 |
|
C1 |
C2 |
H10 |
109.000 |
| C1 |
C2 |
H11 |
109.000 |
|
C1 |
C3 |
O7 |
112.000 |
| C1 |
C3 |
H12 |
109.000 |
|
C1 |
C3 |
H13 |
109.000 |
| C2 |
C1 |
C3 |
112.000 |
|
C2 |
C1 |
H4 |
109.000 |
| C2 |
C1 |
H5 |
109.000 |
|
C2 |
O6 |
H8 |
109.000 |
| C3 |
C1 |
H4 |
109.000 |
|
C3 |
C1 |
H5 |
109.000 |
| C3 |
O7 |
H9 |
109.000 |
|
H4 |
C1 |
H5 |
108.788 |
| O6 |
C2 |
H10 |
111.005 |
|
O6 |
C2 |
H11 |
111.005 |
| O7 |
C3 |
H12 |
109.000 |
|
O7 |
C3 |
H13 |
109.000 |
| H10 |
C2 |
H11 |
108.788 |
|
H12 |
C3 |
H13 |
108.788 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| H-C |
6 |
| H-O |
2 |
| C-C |
2 |
| C-O |
2 |
Connectivity
| Atom 1 |
Atom 2 |
| C1 |
C2 |
| C1 |
C3 |
| C1 |
H4 |
| C1 |
H5 |
| C2 |
O6 |
| C2 |
H10 |
| C2 |
H11 |
| C3 |
O7 |
| C3 |
H12 |
| C3 |
H13 |
| O6 |
H8 |
| O7 |
H9 |










