|
Computational Chemistry Comparison and Benchmark DataBase
Release 22 (May 2022) Standard Reference Database 101
National Institute of Standards and Technology
|
|
|
|
You are here: Home > Geometry > Calculated > Calculated geometry OR Calculated > Geometry > Calculated geometry
|
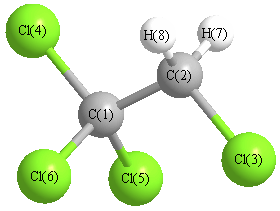
Geometry for CH2ClCCl3 (1,1,1,2-tetrachloroethane)
1A' CS
1910171554
InChI=1S/C2H2Cl4/c3-1-2(4,5)6/h1H2 INChIKey=
QCISD/6-31G(2df,p)
Point group is Cs
| Atom |
Internal |
|
Principal |
| x (Å) |
y (Å) |
z (Å) |
|
a (Å) |
b (Å) |
c (Å) |
| C1 |
0.2684 |
0.2844 |
0.0000 |
|
0.3885 |
0.0000 |
0.0446 |
| C2 |
-1.2601 |
0.3695 |
0.0000 |
|
-0.7259 |
0.0000 |
1.0943 |
| Cl3 |
-2.0466 |
-1.2209 |
0.0000 |
|
-2.3519 |
0.0000 |
0.3844 |
| Cl4 |
0.8606 |
1.9673 |
0.0000 |
|
1.9255 |
0.0000 |
0.9505 |
| Cl5 |
0.8606 |
-0.5419 |
1.4511 |
|
0.3093 |
1.4511 |
-0.9689 |
| Cl6 |
0.8606 |
-0.5419 |
-1.4511 |
|
0.3093 |
-1.4511 |
-0.9689 |
| H7 |
-1.5749 |
0.9064 |
0.8931 |
|
-0.6209 |
0.8931 |
1.7078 |
| H8 |
-1.5749 |
0.9064 |
-0.8931 |
|
-0.6209 |
-0.8931 |
1.7078 |
Atom - Atom Distances (Å)
| |
C1 |
C2 |
Cl3 |
Cl4 |
Cl5 |
Cl6 |
H7 |
H8 |
| C1 |
|
1.5309 |
2.7614 |
1.7841 |
1.7717 |
1.7717 |
2.1407 |
2.1407 |
|---|
| C2 |
1.5309 |
| 1.7742 |
2.6553 |
2.7265 |
2.7265 |
1.0886 |
1.0886 |
|---|
| Cl3 |
2.7614 |
1.7742 |
| 4.3148 |
3.3195 |
3.3195 |
2.3549 |
2.3549 |
|---|
| Cl4 |
1.7841 |
2.6553 |
4.3148 |
| 2.8986 |
2.8986 |
2.8027 |
2.8027 |
|---|
| Cl5 |
1.7717 |
2.7265 |
3.3195 |
2.8986 |
| 2.9022 |
2.8881 |
3.6776 |
|---|
| Cl6 |
1.7717 |
2.7265 |
3.3195 |
2.8986 |
2.9022 |
| 3.6776 |
2.8881 |
|---|
| H7 |
2.1407 |
1.0886 |
2.3549 |
2.8027 |
2.8881 |
3.6776 |
| 1.7861 |
|---|
| H8 |
2.1407 |
1.0886 |
2.3549 |
2.8027 |
3.6776 |
2.8881 |
1.7861 |
|
|---|
Maximum atom distance is 4.3148Å
between atoms Cl3 and Cl4.
Calculated Bond Angles (degrees) (Ignoring Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
C2 |
Cl3 |
113.128 |
|
C2 |
C1 |
Cl4 |
106.199 |
|
C2 |
C1 |
Cl5 |
111.079 |
|
C2 |
C1 |
Cl6 |
111.079 |
|
Cl4 |
C1 |
Cl5 |
109.207 |
|
Cl4 |
C1 |
Cl6 |
109.207 |
|
Cl5 |
C1 |
Cl6 |
109.971 |
|
Calculated Bond Angles (degrees) (Involving Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
C2 |
H7 |
108.432 |
|
C1 |
C2 |
H8 |
108.432 |
|
Cl3 |
C2 |
H7 |
108.295 |
|
Cl3 |
C2 |
H8 |
108.295 |
|
H7 |
C2 |
H8 |
110.253 |
|
For information on specific bond angles or dihedrals
see the geometry comparison page in section
Comparisons > Geometry > Bonds, angles.