|
Computational Chemistry Comparison and Benchmark DataBase
Release 22 (May 2022) Standard Reference Database 101
National Institute of Standards and Technology
|
|
|
|
You are here: Resources > Tutorials > Entropy > Entropy and conformations
|
Configuration changes and contributions to entropy
The rotational contribution to the entropy depends on the
product of the moments of inertia.
How much does this contribution change as the configuration of a molecule changes?
We examine two molecules here: 1,2-dichloroethane (CH2ClCH2Cl)
and hexane (C6H14).
We use energies and rotational constants from HF/6-31G* calculations.
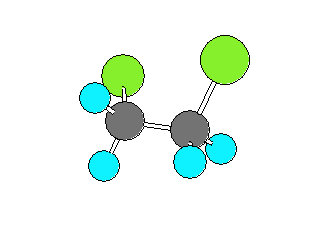
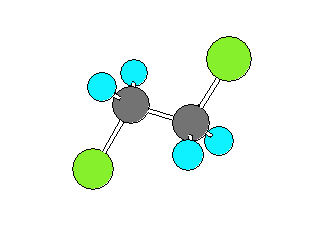
1,2-dichloroethane (CH2ClCH2Cl)
can be in a gauche form (Cl-C-C-Cl dihedral angle of +- 60 degrees)
or a trans form (Cl-C-C-Cl dihedral angle of 180 degrees).
The trans form has the Chlorine and Carbon atoms closer to collinear
which results in the larger rotational constant and smaller product of the moments of inertia.
| gauche | trans |
|---|
 |
 |
Results from HF/6-31G* calculations for a temperature of 298.15 K
| |
gauche | trans | units |
| Rotational constants |
A
B
C |
0.34194
0.07273
0.06372 |
0.98541
0.05003
0.04847 |
cm-1 |
|---|
| Product of moments of inertia |
3.023 |
2.005 |
106 amu3Å6 |
|---|
| Entropy |
Srot |
104.79 |
103.08 |
J K-1 mol-1 |
| Relative energy |
8 |
0 |
kJ mol-1 |
|---|
see section III.A.9 Barriers to internal rotation
for more data on 1,2-dichloroethane.
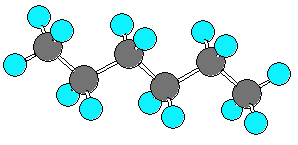
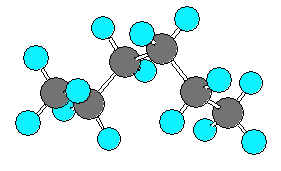
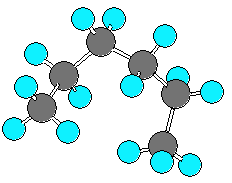
hexane (C6H14) can be in an extended form
or various forms which are more curled up.
There are three C-C-C-C dihedral angles in hexane.
In the extended form all three dihedral angles are 180 degrees.
In the curled (helical) form all three angles are 60 degrees.
In the very curled form the dihedral angles are +60, +60, -90.
The last angle is different from -60 due to steric repulsion.
| extended |
curled (helical) |
very curled |
 |
 |
 |
Results from HF/6-31G* calculations for a temperature of 298.15 K
| |
extended |
curled |
very curled |
units |
| Rotational constants |
A
B
C |
0.49165
0.03786
0.03655 |
0.19887
0.05790
0.05776 |
0.15470
0.07193
0.05803 |
cm-1 |
|---|
| Product of moments of inertia |
7.041748 |
7.202027 |
7.418987 |
106 amu3Å6 |
|---|
| Entropy |
Srot |
108.31 |
108.40 |
108.52 |
J K-1 mol-1 |
| Relative energy |
0 |
12 |
21 |
kJ mol-1 |
see section III.A.9 Barriers to internal rotation
for data on rotation about the central bond in butane (C4H10),
which should have a potential energy surface for internal rotation similar to hexane.
For calculating ideal-gas thermochemical properties see section
I.D. A brief description of the thermochemical quantities and methods.




