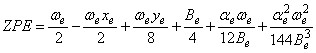Vibrational Zero-Point Energies (ZPE)
The vibrational zero-point energy is the energy difference between the lowest point on the potential energy surface (equilibrium energy)
and the energy of the vibrationless energy level (v=0).
It is not possible to measure the ZPE.
The ZPE can be approximated as half the fundamental vibrational frequencies.
This assumes the vibrational frequencies are harmonic and there are no vibration-rotation interactions.
Given more vibrational and rotational details,
such as anharmonic contants (ωexe, ωeye, ...),
vibration-ration interaction constants (αe) better approximations to the ZPE can be made.
For diatomics we use:
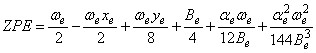
See the paper by Irikura: K. K. Irikura "Experimental Vibrational Zero-Point Energies: Diatomic Molecules" J. Phys. Chem. Ref. Data 36(2), 389, 2007