|
Computational Chemistry Comparison and Benchmark DataBase
Release 22 (May 2022) Standard Reference Database 101
National Institute of Standards and Technology
|
|
|
|
You are here: Calculated > Vibrations > Scale Factors > Why scale vibrations OR Resources > Tutorials > Vibrations > Why scale vibrations
|
Vibrational scaling factors
The vibrational frequencies produced by ab initio programs are often
multiplied by a scale factor (in the range of 0.8 to 1.0) to better match
experimental vibrational frequencies. This scaling compensates for two problems:
1) The electronic structure calculation is approximate.
Usually less than a relativistic full configuration interaction is performed.
2) The potential energy surface is not harmonic.
For bond stretches a better description of the potential energy surface
is given by the Morse potential
E(x) = D(1-exp(-β( x-x0 )))2
illustrated below.
In this equation E is the potential energy,
D, β, and x0 are constants,
and x is the interatomic distance.
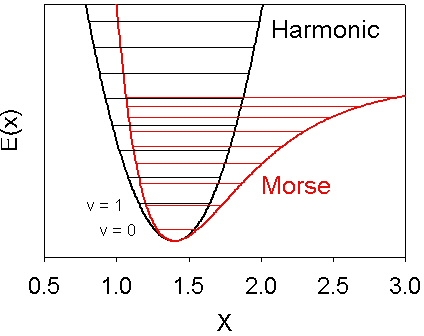
The programs that predict vibrational frequencies do so by calculating
the second derivative of the potential energy surface with respect to the atomic
coordinates. This provides the curvature at the bottom (minimum) of the well.
For a harmonic potential E(x) = k x 2 this is directly related to the vibrational
energy level spacing. For a Morse potential (with the same second derivative at
the minimum) the anharmonicity causes the vibrational energy levels to be more
closely spaced as illustrated in the figure.
Experimentally usually the fundamental (v=0 to v=1 energy) is measured. If
enough vibrational levels are observed a harmonic frequency can be estimated
with a formula such as
G(v) = ωe(v+1/2)
- ωexe(v+1/2)2
+ ωeye(v+1/2)3 + ...
where G(v) is the vibrational energy, v is the vibrational quantum number,
ωe is the harmonic frequency,
and ωexe
and ωeye are anharmonic constants. For polyatomic molecules
usually only the fundamental is experimentally observed.
We use the experimentally observed vibrational frequencies
(νi),
and the theoretical vibrational frequencies
(ωi).
The scaling factor (c) and its relative uncertainty (ur)
are obtained from the following sums over the vibrational frequencies:
c = Σ(νi •
ωi)/
Σ(ωi2)
ur2 = (Σ(ωi2
• (c-νi/ωi)2))/(Σ(ωi2))
Try out the CCCBDB
pages for calculating vibrational scaling factors.
How much do the factors change with basis set?
The following figure shows several scaling factors and their uncertainties. The figure is from:
K. K. Irikura, R. D. Johnson III, R. N. Kacker,
J. Phys. Chem. A.,
2005, 109(37), 8430-8437.
Filled cirlces = HF, filled squares = MP2, open cirlces = BLYP, open squares = B3LYP.
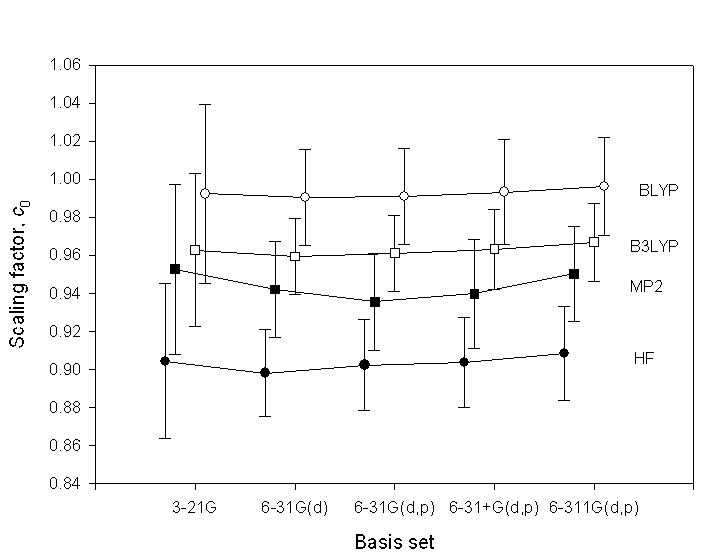
The scaling factors depend weakly on basis set.
The uncertainties are about twice as large for the 3-21G
basis set as for the other basis sets, all of which include
polarization functions. This suggests that polarization functions
are important for avoiding markedly poor predictions of
vibrational frequencies.
For larger basis sets the scaling factors and uncertainties have non-significant changes.
Thus the same scaling factors can be used for other basis sets.
See the pages listing vibrational scaling factors: Calculated; Vibrations; Scale Factors; Scale factors

