Jump to
S1C2
Energy calculated at MP2/6-31G**
| | hartrees |
|---|
| Energy at 0K | -112.773659 |
| Energy at 298.15K | -112.779149 |
| HF Energy | -112.395007 |
| Counterpoise corrected energy | |
| CP Energy at 298.15K | |
| Counterpoise optimized geometry corrected energy | |
| CP opt. Energy at 298.15K | |
| Nuclear repulsion energy | 40.486611 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP2/6-31G**
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
Ag |
3704 |
3469 |
0.00 |
|
|
|
| 2 |
Ag |
3548 |
3323 |
0.00 |
|
|
|
| 3 |
Ag |
1733 |
1623 |
0.00 |
|
|
|
| 4 |
Ag |
1161 |
1088 |
0.00 |
|
|
|
| 5 |
Ag |
474 |
444 |
0.00 |
|
|
|
| 6 |
Ag |
152 |
142 |
0.00 |
|
|
|
| 7 |
Au |
3713 |
3477 |
0.81 |
|
|
|
| 8 |
Au |
1748 |
1637 |
29.16 |
|
|
|
| 9 |
Au |
255 |
239 |
122.79 |
|
|
|
| 10 |
Au |
104 |
97 |
27.45 |
|
|
|
| 11 |
Bg |
3713 |
3477 |
0.00 |
|
|
|
| 12 |
Bg |
1736 |
1626 |
0.00 |
|
|
|
| 13 |
Bg |
142 |
133 |
0.00 |
|
|
|
| 14 |
Bu |
3704 |
3469 |
25.41 |
|
|
|
| 15 |
Bu |
3553 |
3327 |
15.14 |
|
|
|
| 16 |
Bu |
1708 |
1599 |
17.08 |
|
|
|
| 17 |
Bu |
1138 |
1066 |
365.24 |
|
|
|
| 18 |
Bu |
108 |
102 |
301.39 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 16196.6 cm
-1
Scaled (by 0.9365) Zero Point Vibrational Energy (zpe) 15168.1 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at MP2/6-31G**
Point Group is C2h
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| H1 |
0.661 |
0.798 |
0.000 |
| N2 |
0.000 |
1.567 |
0.000 |
| N3 |
0.000 |
-1.567 |
0.000 |
| H4 |
0.218 |
2.135 |
0.809 |
| H5 |
0.218 |
2.135 |
-0.809 |
| H6 |
-0.661 |
-0.798 |
0.000 |
| H7 |
-0.218 |
-2.135 |
-0.809 |
| H8 |
-0.218 |
-2.135 |
0.809 |
Atom - Atom Distances (Å)
| |
H1 |
N2 |
N3 |
H4 |
H5 |
H6 |
H7 |
H8 |
| H1 | | 1.0140 | 2.4558 | 1.6246 | 1.6246 | 2.0723 | 3.1676 | 3.1676 |
N2 | 1.0140 | | 3.1344 | 1.0128 | 1.0128 | 2.4558 | 3.7963 | 3.7963 | N3 | 2.4558 | 3.1344 | | 3.7963 | 3.7963 | 1.0140 | 1.0128 | 1.0128 | H4 | 1.6246 | 1.0128 | 3.7963 | | 1.6188 | 3.1676 | 4.5882 | 4.2931 | H5 | 1.6246 | 1.0128 | 3.7963 | 1.6188 | | 3.1676 | 4.2931 | 4.5882 | H6 | 2.0723 | 2.4558 | 1.0140 | 3.1676 | 3.1676 | | 1.6246 | 1.6246 | H7 | 3.1676 | 3.7963 | 1.0128 | 4.5882 | 4.2931 | 1.6246 | | 1.6188 | H8 | 3.1676 | 3.7963 | 1.0128 | 4.2931 | 4.5882 | 1.6246 | 1.6188 | |
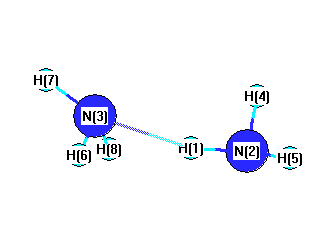 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| H1 |
N2 |
H4 |
106.567 |
|
H1 |
N2 |
H5 |
106.567 |
| H1 |
H3 |
N6 |
56.276 |
|
H1 |
H3 |
H7 |
126.755 |
| H1 |
H3 |
H8 |
126.755 |
|
N2 |
H1 |
H3 |
123.724 |
| H4 |
N2 |
H5 |
106.107 |
|
N6 |
H3 |
H7 |
106.567 |
| N6 |
H3 |
H8 |
106.567 |
|
H7 |
H3 |
H8 |
106.107 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at MP2/6-31G**
| | hartrees |
|---|
| Energy at 0K | -112.773659 |
| Energy at 298.15K | -112.779137 |
| HF Energy | -112.395010 |
| Counterpoise corrected energy | |
| CP Energy at 298.15K | |
| Counterpoise optimized geometry corrected energy | |
| CP opt. Energy at 298.15K | |
| Nuclear repulsion energy | 40.489584 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP2/6-31G**
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3704 |
3469 |
25.10 |
|
|
|
| 2 |
A' |
3704 |
3469 |
0.25 |
|
|
|
| 3 |
A' |
3553 |
3328 |
15.27 |
|
|
|
| 4 |
A' |
3549 |
3323 |
0.00 |
|
|
|
| 5 |
A' |
1733 |
1623 |
0.00 |
|
|
|
| 6 |
A' |
1708 |
1599 |
17.08 |
|
|
|
| 7 |
A' |
1161 |
1087 |
0.00 |
|
|
|
| 8 |
A' |
1138 |
1066 |
365.41 |
|
|
|
| 9 |
A' |
474 |
444 |
0.00 |
|
|
|
| 10 |
A' |
152 |
142 |
0.00 |
|
|
|
| 11 |
A' |
107 |
100 |
301.43 |
|
|
|
| 12 |
A" |
3714 |
3478 |
0.82 |
|
|
|
| 13 |
A" |
3713 |
3478 |
0.00 |
|
|
|
| 14 |
A" |
1748 |
1637 |
29.17 |
|
|
|
| 15 |
A" |
1736 |
1626 |
0.00 |
|
|
|
| 16 |
A" |
254 |
238 |
122.34 |
|
|
|
| 17 |
A" |
140 |
131 |
0.00 |
|
|
|
| 18 |
A" |
100 |
94 |
27.86 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 16194.0 cm
-1
Scaled (by 0.9365) Zero Point Vibrational Energy (zpe) 15165.7 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at MP2/6-31G**
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| H1 |
0.167 |
0.694 |
0.000 |
| N2 |
-0.027 |
1.681 |
0.000 |
| N3 |
-0.027 |
-1.589 |
0.000 |
| H4 |
0.354 |
2.117 |
0.816 |
| H5 |
0.354 |
2.117 |
-0.816 |
| H6 |
-1.019 |
-1.438 |
0.000 |
| H7 |
0.265 |
-2.068 |
-0.831 |
| H8 |
0.265 |
-2.068 |
0.831 |
Atom - Atom Distances (Å)
| |
H1 |
N2 |
N3 |
H4 |
H5 |
H6 |
H7 |
H8 |
| H1 | | 1.0062 | 2.2907 | 1.6512 | 1.6512 | 2.4395 | 2.8853 | 2.8853 |
N2 | 1.0062 | | 3.2699 | 1.0006 | 1.0006 | 3.2731 | 3.8508 | 3.8508 | N3 | 2.2907 | 3.2699 | | 3.8136 | 3.8136 | 1.0032 | 1.0027 | 1.0027 | H4 | 1.6512 | 1.0006 | 3.8136 | | 1.6319 | 3.8974 | 4.4978 | 4.1855 | H5 | 1.6512 | 1.0006 | 3.8136 | 1.6319 | | 3.8974 | 4.1855 | 4.4978 | H6 | 2.4395 | 3.2731 | 1.0032 | 3.8974 | 3.8974 | | 1.6542 | 1.6542 | H7 | 2.8853 | 3.8508 | 1.0027 | 4.4978 | 4.1855 | 1.6542 | | 1.6620 | H8 | 2.8853 | 3.8508 | 1.0027 | 4.1855 | 4.4978 | 1.6542 | 1.6620 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| H1 |
N2 |
H4 |
110.731 |
|
H1 |
N2 |
H5 |
110.731 |
| H1 |
H3 |
N6 |
86.221 |
|
H1 |
H3 |
H7 |
116.811 |
| H1 |
H3 |
H8 |
116.811 |
|
N2 |
H1 |
H3 |
164.023 |
| H4 |
N2 |
H5 |
109.271 |
|
N6 |
H3 |
H7 |
111.111 |
| N6 |
H3 |
H8 |
111.111 |
|
H7 |
H3 |
H8 |
111.947 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
