Vibrational Frequencies calculated at PBEPBEultrafine/6-31+G**
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
3806 |
3763 |
86.75 |
|
|
|
| 2 |
A |
3567 |
3526 |
279.71 |
|
|
|
| 3 |
A |
2941 |
2907 |
72.91 |
|
|
|
| 4 |
A |
2862 |
2829 |
67.26 |
|
|
|
| 5 |
A |
1746 |
1726 |
90.29 |
|
|
|
| 6 |
A |
1588 |
1570 |
100.29 |
|
|
|
| 7 |
A |
1483 |
1466 |
16.61 |
|
|
|
| 8 |
A |
1228 |
1214 |
5.00 |
|
|
|
| 9 |
A |
1155 |
1142 |
7.65 |
|
|
|
| 10 |
A |
530 |
524 |
115.40 |
|
|
|
| 11 |
A |
377 |
372 |
144.28 |
|
|
|
| 12 |
A |
193 |
191 |
30.45 |
|
|
|
| 13 |
A |
181 |
179 |
0.07 |
|
|
|
| 14 |
A |
80 |
79 |
48.03 |
|
|
|
| 15 |
A |
50 |
49 |
173.63 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 10892.5 cm
-1
Scaled (by 0.9886) Zero Point Vibrational Energy (zpe) 10768.4 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Charges, Dipole, Quadrupole and Polarizability
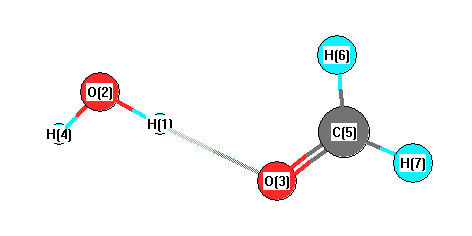
Charges from optimized geometry at PBEPBEultrafine/6-31+G**
Charges (e)
| Number |
Element |
Mulliken |
CHELPG |
AIM |
ESP |
| 1 |
H |
0.374 |
|
|
|
| 2 |
O |
-0.756 |
|
|
|
| 3 |
O |
-0.302 |
|
|
|
| 4 |
H |
0.356 |
|
|
|
| 5 |
C |
0.022 |
|
|
|
| 6 |
H |
0.165 |
|
|
|
| 7 |
H |
0.139 |
|
|
|
Electric dipole moments
Electric dipole components in Debye
(What's a Debye? See section
VII.A.3)
| |
x |
y |
z |
Total |
| |
1.346 |
0.266 |
0.003 |
1.372 |
| CHELPG |
|
|
|
|
| AIM |
|
|
|
|
| ESP |
|
|
|
|
Electric Quadrupole moment
Quadrupole components in D Å
Polarizabilities
Components of the polarizability tensor.
Units are
Å
3 (Angstrom cubed)
Change units.
| |
x |
y |
z |
| x |
0.000 |
0.000 |
0.000 |
| y |
0.000 |
0.000 |
0.000 |
| z |
0.000 |
0.000 |
0.000 |
<r2> (average value of r
2) Å
2
| <r2> |
0.000 |
| (<r2>)1/2 |
0.000 |