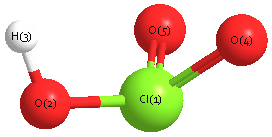Jump to
S1C2
Energy calculated at CCD/6-31G
| | hartrees |
|---|
| Energy at 0K | -684.533074 |
| Energy at 298.15K | |
| HF Energy | -684.096114 |
| Nuclear repulsion energy | 168.973952 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCD/6-31G
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3498 |
3356 |
49.08 |
|
|
|
| 2 |
A' |
1196 |
1148 |
80.61 |
|
|
|
| 3 |
A' |
583 |
560 |
0.18 |
|
|
|
| 4 |
A' |
486 |
467 |
96.23 |
|
|
|
| 5 |
A' |
304 |
291 |
36.77 |
|
|
|
| 6 |
A' |
249 |
239 |
12.05 |
|
|
|
| 7 |
A" |
667 |
640 |
12.55 |
|
|
|
| 8 |
A" |
286 |
274 |
91.76 |
|
|
|
| 9 |
A" |
114i |
110i |
82.37 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 3577.3 cm
-1
Scaled (by 0.9595) Zero Point Vibrational Energy (zpe) 3432.5 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CCD/6-31G
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
0.422 |
0.109 |
0.000 |
| O2 |
-0.247 |
-1.648 |
0.000 |
| H3 |
-1.235 |
-1.524 |
0.000 |
| O4 |
-0.247 |
0.804 |
1.420 |
| O5 |
-0.247 |
0.804 |
-1.420 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.8802 | 2.3259 | 1.7170 | 1.7170 |
O2 | 1.8802 | | 0.9953 | 2.8340 | 2.8340 | H3 | 2.3259 | 0.9953 | | 2.9002 | 2.9002 | O4 | 1.7170 | 2.8340 | 2.9002 | | 2.8399 | O5 | 1.7170 | 2.8340 | 2.9002 | 2.8399 | |
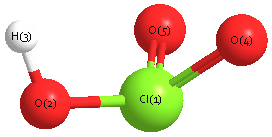 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
36.027 |
|
O2 |
Cl1 |
O3 |
24.571 |
| O2 |
Cl1 |
O4 |
103.878 |
|
O3 |
Cl1 |
O4 |
90.384 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at CCD/6-31G
| | hartrees |
|---|
| Energy at 0K | -684.533348 |
| Energy at 298.15K | |
| HF Energy | -684.096627 |
| Nuclear repulsion energy | 169.030216 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCD/6-31G
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
3482 |
3341 |
54.83 |
|
|
|
| 2 |
A |
1207 |
1158 |
86.18 |
|
|
|
| 3 |
A |
671 |
644 |
10.59 |
|
|
|
| 4 |
A |
580 |
557 |
0.26 |
|
|
|
| 5 |
A |
494 |
474 |
99.87 |
|
|
|
| 6 |
A |
318 |
305 |
115.12 |
|
|
|
| 7 |
A |
296 |
284 |
50.16 |
|
|
|
| 8 |
A |
245 |
235 |
11.23 |
|
|
|
| 9 |
A |
151 |
145 |
49.23 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 3722.5 cm
-1
Scaled (by 0.9595) Zero Point Vibrational Energy (zpe) 3571.7 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CCD/6-31G
Point Group is C1
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
-0.156 |
0.049 |
-0.412 |
| O2 |
1.303 |
-1.016 |
0.114 |
| H3 |
1.645 |
-0.510 |
0.901 |
| O4 |
0.354 |
1.559 |
0.277 |
| O5 |
-1.531 |
-0.583 |
0.372 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.8813 | 2.2983 | 1.7368 | 1.7041 |
O2 | 1.8813 | | 0.9966 | 2.7488 | 2.8790 | H3 | 2.2983 | 0.9966 | | 2.5170 | 3.2214 | O4 | 1.7368 | 2.7488 | 2.5170 | | 2.8550 | O5 | 1.7041 | 2.8790 | 3.2214 | 2.8550 | |
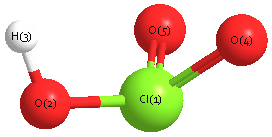 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
34.531 |
|
O2 |
Cl1 |
O3 |
25.143 |
| O2 |
Cl1 |
O4 |
98.802 |
|
O3 |
Cl1 |
O4 |
75.763 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability