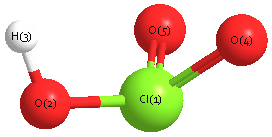Jump to
S1C2
Energy calculated at CID/6-31G
| | hartrees |
|---|
| Energy at 0K | -684.478540 |
| Energy at 298.15K | |
| HF Energy | -684.105214 |
| Nuclear repulsion energy | 168.921443 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CID/6-31G
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3617 |
3382 |
101.69 |
|
|
|
| 2 |
A' |
1285 |
1202 |
52.74 |
|
|
|
| 3 |
A' |
629 |
588 |
28.43 |
|
|
|
| 4 |
A' |
481 |
450 |
10.16 |
|
|
|
| 5 |
A' |
275 |
257 |
35.62 |
|
|
|
| 6 |
A' |
241 |
225 |
14.52 |
|
|
|
| 7 |
A" |
600 |
562 |
0.12 |
|
|
|
| 8 |
A" |
302 |
282 |
106.55 |
|
|
|
| 9 |
A" |
107i |
100i |
97.36 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 3661.1 cm
-1
Scaled (by 0.9352) Zero Point Vibrational Energy (zpe) 3423.9 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CID/6-31G
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
-0.368 |
0.231 |
0.000 |
| O2 |
1.278 |
0.994 |
0.000 |
| H3 |
1.906 |
0.233 |
0.000 |
| O4 |
-0.368 |
-0.757 |
1.446 |
| O5 |
-0.368 |
-0.757 |
-1.446 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.8145 | 2.2737 | 1.7511 | 1.7511 |
O2 | 1.8145 | | 0.9863 | 2.8049 | 2.8049 | H3 | 2.2737 | 0.9863 | | 2.8707 | 2.8707 | O4 | 1.7511 | 2.8049 | 2.8707 | | 2.8912 | O5 | 1.7511 | 2.8049 | 2.8707 | 2.8912 | |
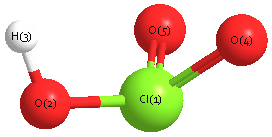 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
37.334 |
|
O2 |
Cl1 |
O3 |
24.816 |
| O2 |
Cl1 |
O4 |
103.732 |
|
O3 |
Cl1 |
O4 |
90.033 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at CID/6-31G
| | hartrees |
|---|
| Energy at 0K | -684.478754 |
| Energy at 298.15K | |
| HF Energy | -684.105436 |
| Nuclear repulsion energy | 169.001076 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CID/6-31G
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
3597 |
3363 |
106.63 |
|
|
|
| 2 |
A |
1293 |
1210 |
56.84 |
|
|
|
| 3 |
A |
634 |
593 |
27.42 |
|
|
|
| 4 |
A |
604 |
565 |
2.08 |
|
|
|
| 5 |
A |
482 |
451 |
13.99 |
|
|
|
| 6 |
A |
322 |
301 |
153.54 |
|
|
|
| 7 |
A |
280 |
262 |
24.66 |
|
|
|
| 8 |
A |
237 |
221 |
8.60 |
|
|
|
| 9 |
A |
140 |
131 |
61.44 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 3793.9 cm
-1
Scaled (by 0.9352) Zero Point Vibrational Energy (zpe) 3548.1 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CID/6-31G
Point Group is C1
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
-0.123 |
0.062 |
-0.419 |
| O2 |
1.014 |
-1.244 |
0.129 |
| H3 |
1.432 |
-0.847 |
0.932 |
| O4 |
0.715 |
1.455 |
0.276 |
| O5 |
-1.645 |
-0.237 |
0.368 |
Atom - Atom Distances (Å)
| |
Cl1 |
O2 |
H3 |
O4 |
O5 |
| Cl1 | | 1.8165 | 2.2515 | 1.7677 | 1.7391 |
O2 | 1.8165 | | 0.9877 | 2.7190 | 2.8534 | H3 | 2.2515 | 0.9877 | | 2.4984 | 3.1872 | O4 | 1.7677 | 2.7190 | 2.4984 | | 2.9048 | O5 | 1.7391 | 2.8534 | 3.1872 | 2.9048 | |
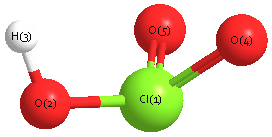 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Cl1 |
O2 |
H5 |
35.714 |
|
O2 |
Cl1 |
O3 |
25.329 |
| O2 |
Cl1 |
O4 |
98.673 |
|
O3 |
Cl1 |
O4 |
75.801 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability