Jump to
S1C2
Energy calculated at MP2=FULL/6-31+G**
| | hartrees |
|---|
| Energy at 0K | -130.745308 |
| Energy at 298.15K | -130.747354 |
| HF Energy | -130.403158 |
| Nuclear repulsion energy | 35.167862 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP2=FULL/6-31+G**
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A1 |
3544 |
3329 |
15.90 |
|
|
|
| 2 |
A1 |
1719 |
1615 |
15.00 |
|
|
|
| 3 |
A1 |
1515 |
1422 |
20.63 |
|
|
|
| 4 |
B1 |
146 |
137 |
211.06 |
|
|
|
| 5 |
B2 |
3706 |
3481 |
21.18 |
|
|
|
| 6 |
B2 |
1284 |
1206 |
3.53 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 5956.7 cm
-1
Scaled (by 0.9392) Zero Point Vibrational Energy (zpe) 5594.5 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
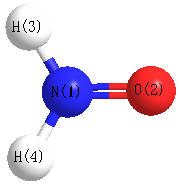
Geometric Data calculated at MP2=FULL/6-31+G**
Point Group is C2v
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| N1 |
0.000 |
0.000 |
-0.540 |
| O2 |
0.000 |
0.000 |
0.734 |
| H3 |
0.000 |
0.876 |
-1.046 |
| H4 |
0.000 |
-0.876 |
-1.046 |
Atom - Atom Distances (Å)
| |
N1 |
O2 |
H3 |
H4 |
| N1 | | 1.2731 | 1.0121 | 1.0121 |
O2 | 1.2731 | | 1.9835 | 1.9835 | H3 | 1.0121 | 1.9835 | | 1.7527 | H4 | 1.0121 | 1.9835 | 1.7527 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| O2 |
N1 |
H3 |
120.017 |
|
O2 |
N1 |
H4 |
120.017 |
| H3 |
N1 |
H4 |
119.966 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at MP2=FULL/6-31+G**
| | hartrees |
|---|
| Energy at 0K | -130.744481 |
| Energy at 298.15K | |
| HF Energy | -130.404205 |
| Nuclear repulsion energy | 35.314867 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP2=FULL/6-31+G**
Geometric Data calculated at MP2=FULL/6-31+G**
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| N1 |
-0.025 |
0.543 |
0.000 |
| O2 |
-0.025 |
-0.730 |
0.000 |
| H3 |
0.186 |
1.021 |
0.853 |
| H4 |
0.186 |
1.021 |
-0.853 |
Atom - Atom Distances (Å)
| |
N1 |
O2 |
H3 |
H4 |
| N1 | | 1.2731 | 1.0002 | 1.0002 |
O2 | 1.2731 | | 1.9594 | 1.9594 | H3 | 1.0002 | 1.9594 | | 1.7054 | H4 | 1.0002 | 1.9594 | 1.7054 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| O2 |
N1 |
H3 |
118.577 |
|
O2 |
N1 |
H4 |
118.577 |
| H3 |
N1 |
H4 |
116.982 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
