All results from a given calculation for H2ONH3 (Water Ammonia Dimer)
using model chemistry: MP2/6-31+G**
19 10 17 12 22
States and conformations
| State |
Conformation |
minimum conformation |
conformer description |
state description |
| 1 |
1 |
yes |
C1 |
1A' |
Energy calculated at MP2/6-31+G**
| | hartrees |
|---|
| Energy at 0K | -132.564439 |
| Energy at 298.15K | |
| HF Energy | -132.203856 |
| Counterpoise corrected energy | -132.635009 |
| CP Energy at 298.15K | -132.639278 |
| Counterpoise optimized geometry corrected energy | |
| CP opt. Energy at 298.15K | |
| Nuclear repulsion energy | 38.563527 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP2/6-31+G**
Geometric Data calculated at MP2/6-31+G**
Point Group is Cs
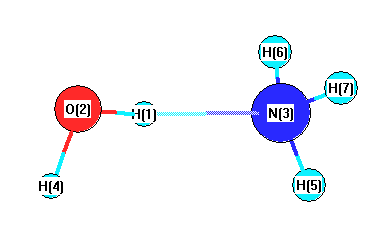
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| H1 |
0.013 |
0.597 |
0.000 |
| O2 |
-0.037 |
1.576 |
0.000 |
| N3 |
-0.037 |
-1.393 |
0.000 |
| H4 |
0.889 |
1.856 |
0.000 |
| H5 |
0.809 |
-1.958 |
0.000 |
| H6 |
-0.577 |
-1.674 |
0.816 |
| H7 |
-0.577 |
-1.674 |
-0.816 |
Atom - Atom Distances (Å)
| |
H1 |
O2 |
N3 |
H4 |
H5 |
H6 |
H7 |
| H1 | | 0.9804 | 1.9908 | 1.5339 | 2.6764 | 2.4835 | 2.4835 |
O2 | 0.9804 | | 2.9694 | 0.9675 | 3.6342 | 3.3936 | 3.3936 | N3 | 1.9908 | 2.9694 | | 3.3786 | 1.0174 | 1.0174 | 1.0174 | H4 | 1.5339 | 0.9675 | 3.3786 | | 3.8150 | 3.9077 | 3.9077 | H5 | 2.6764 | 3.6342 | 1.0174 | 3.8150 | | 1.6330 | 1.6330 | H6 | 2.4835 | 3.3936 | 1.0174 | 3.9077 | 1.6330 | | 1.6317 | H7 | 2.4835 | 3.3936 | 1.0174 | 3.9077 | 1.6330 | 1.6317 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| H1 |
O2 |
H4 |
103.897 |
|
H1 |
N3 |
H5 |
122.294 |
| H1 |
N3 |
H6 |
106.773 |
|
H1 |
N3 |
H7 |
106.773 |
| O2 |
H1 |
N3 |
175.651 |
|
H5 |
N3 |
H6 |
106.742 |
| H5 |
N3 |
H7 |
106.742 |
|
H6 |
N3 |
H7 |
106.621 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
