Jump to
S1C2
Energy calculated at MP2/cc-pVDZ
| | hartrees |
|---|
| Energy at 0K | -1194.153159 |
| Energy at 298.15K | -1194.155525 |
| HF Energy | -1193.756234 |
| Nuclear repulsion energy | 193.878034 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP2/cc-pVDZ
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
2741 |
2611 |
0.42 |
|
|
|
| 2 |
A |
887 |
845 |
0.01 |
|
|
|
| 3 |
A |
499 |
476 |
0.56 |
|
|
|
| 4 |
A |
307 |
292 |
26.23 |
|
|
|
| 5 |
A |
207 |
197 |
0.03 |
|
|
|
| 6 |
B |
2740 |
2610 |
3.47 |
|
|
|
| 7 |
B |
877 |
835 |
5.36 |
|
|
|
| 8 |
B |
495 |
472 |
22.02 |
|
|
|
| 9 |
B |
336 |
320 |
20.47 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4544.3 cm
-1
Scaled (by 0.9525) Zero Point Vibrational Energy (zpe) 4328.5 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
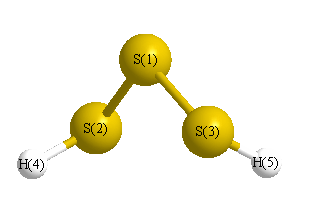
Geometric Data calculated at MP2/cc-pVDZ
Point Group is C2
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| S1 |
0.000 |
0.000 |
0.860 |
| S2 |
0.000 |
1.672 |
-0.393 |
| S3 |
0.000 |
-1.672 |
-0.393 |
| H4 |
-1.336 |
1.743 |
-0.591 |
| H5 |
1.336 |
-1.743 |
-0.591 |
Atom - Atom Distances (Å)
| |
S1 |
S2 |
S3 |
H4 |
H5 |
| S1 | | 2.0893 | 2.0893 | 2.6318 | 2.6318 |
S2 | 2.0893 | | 3.3441 | 1.3522 | 3.6720 | S3 | 2.0893 | 3.3441 | | 3.6720 | 1.3522 | H4 | 2.6318 | 1.3522 | 3.6720 | | 4.3913 | H5 | 2.6318 | 3.6720 | 1.3522 | 4.3913 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| S1 |
S2 |
H4 |
97.453 |
|
S1 |
S3 |
H5 |
97.453 |
| S2 |
S1 |
S3 |
106.313 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at MP2/cc-pVDZ
| | hartrees |
|---|
| Energy at 0K | -1194.152773 |
| Energy at 298.15K | -1194.155150 |
| HF Energy | -1193.755764 |
| Nuclear repulsion energy | 193.908551 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP2/cc-pVDZ
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
2733 |
2603 |
7.66 |
|
|
|
| 2 |
A' |
892 |
850 |
2.98 |
|
|
|
| 3 |
A' |
500 |
476 |
0.55 |
|
|
|
| 4 |
A' |
336 |
320 |
19.74 |
|
|
|
| 5 |
A' |
208 |
198 |
0.07 |
|
|
|
| 6 |
A" |
2735 |
2605 |
0.39 |
|
|
|
| 7 |
A" |
880 |
838 |
3.78 |
|
|
|
| 8 |
A" |
496 |
472 |
24.99 |
|
|
|
| 9 |
A" |
312 |
297 |
11.31 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4545.5 cm
-1
Scaled (by 0.9525) Zero Point Vibrational Energy (zpe) 4329.6 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at MP2/cc-pVDZ
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| S1 |
-0.054 |
0.857 |
0.000 |
| S2 |
-0.054 |
-0.396 |
1.671 |
| S3 |
-0.054 |
-0.396 |
-1.671 |
| H4 |
1.288 |
-0.524 |
1.793 |
| H5 |
1.288 |
-0.524 |
-1.793 |
Atom - Atom Distances (Å)
| |
S1 |
S2 |
S3 |
H4 |
H5 |
| S1 | | 2.0887 | 2.0887 | 2.6307 | 2.6307 |
S2 | 2.0887 | | 3.3423 | 1.3528 | 3.7171 | S3 | 2.0887 | 3.3423 | | 3.7171 | 1.3528 | H4 | 2.6307 | 1.3528 | 3.7171 | | 3.5863 | H5 | 2.6307 | 3.7171 | 1.3528 | 3.5863 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| S1 |
S2 |
H4 |
97.400 |
|
S1 |
S3 |
H5 |
97.400 |
| S2 |
S1 |
S3 |
106.280 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
