Jump to
S1C2
Energy calculated at MP4/6-31G*
| | hartrees |
|---|
| Energy at 0K | -1194.099113 |
| Energy at 298.15K | -1194.101477 |
| HF Energy | -1193.683652 |
| Nuclear repulsion energy | 194.012981 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4/6-31G*
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
2684 |
2563 |
|
|
|
|
| 2 |
A |
915 |
874 |
|
|
|
|
| 3 |
A |
489 |
467 |
|
|
|
|
| 4 |
A |
314 |
300 |
|
|
|
|
| 5 |
A |
208 |
198 |
|
|
|
|
| 6 |
B |
2684 |
2563 |
|
|
|
|
| 7 |
B |
901 |
860 |
|
|
|
|
| 8 |
B |
491 |
469 |
|
|
|
|
| 9 |
B |
345 |
329 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4515.7 cm
-1
Scaled (by 0.9548) Zero Point Vibrational Energy (zpe) 4311.6 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
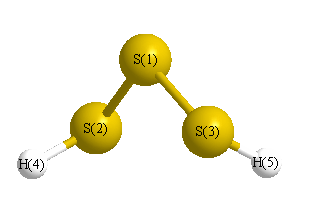
Geometric Data calculated at MP4/6-31G*
Point Group is C2
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| S1 |
0.000 |
0.000 |
0.847 |
| S2 |
0.000 |
1.679 |
-0.387 |
| S3 |
0.000 |
-1.679 |
-0.387 |
| H4 |
-1.331 |
1.783 |
-0.591 |
| H5 |
1.331 |
-1.783 |
-0.591 |
Atom - Atom Distances (Å)
| |
S1 |
S2 |
S3 |
H4 |
H5 |
| S1 | | 2.0832 | 2.0832 | 2.6499 | 2.6499 |
S2 | 2.0832 | | 3.3573 | 1.3511 | 3.7147 | S3 | 2.0832 | 3.3573 | | 3.7147 | 1.3511 | H4 | 2.6499 | 1.3511 | 3.7147 | | 4.4509 | H5 | 2.6499 | 3.7147 | 1.3511 | 4.4509 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| S1 |
S2 |
H4 |
98.755 |
|
S1 |
S3 |
H5 |
98.755 |
| S2 |
S1 |
S3 |
107.378 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at MP4/6-31G*
| | hartrees |
|---|
| Energy at 0K | -1194.098678 |
| Energy at 298.15K | -1194.101058 |
| HF Energy | -1193.683146 |
| Nuclear repulsion energy | 194.058743 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4/6-31G*
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
2675 |
2554 |
|
|
|
|
| 2 |
A' |
917 |
875 |
|
|
|
|
| 3 |
A' |
490 |
468 |
|
|
|
|
| 4 |
A' |
346 |
330 |
|
|
|
|
| 5 |
A' |
209 |
200 |
|
|
|
|
| 6 |
A" |
2676 |
2555 |
|
|
|
|
| 7 |
A" |
908 |
867 |
|
|
|
|
| 8 |
A" |
493 |
471 |
|
|
|
|
| 9 |
A" |
321 |
306 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4517.1 cm
-1
Scaled (by 0.9548) Zero Point Vibrational Energy (zpe) 4312.9 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at MP4/6-31G*
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| S1 |
-0.053 |
0.843 |
0.000 |
| S2 |
-0.053 |
-0.389 |
1.678 |
| S3 |
-0.053 |
-0.389 |
-1.678 |
| H4 |
1.282 |
-0.528 |
1.833 |
| H5 |
1.282 |
-0.528 |
-1.833 |
Atom - Atom Distances (Å)
| |
S1 |
S2 |
S3 |
H4 |
H5 |
| S1 | | 2.0820 | 2.0820 | 2.6505 | 2.6505 |
S2 | 2.0820 | | 3.3565 | 1.3520 | 3.7594 | S3 | 2.0820 | 3.3565 | | 3.7594 | 1.3520 | H4 | 2.6505 | 1.3520 | 3.7594 | | 3.6660 | H5 | 2.6505 | 3.7594 | 1.3520 | 3.6660 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| S1 |
S2 |
H4 |
98.813 |
|
S1 |
S3 |
H5 |
98.813 |
| S2 |
S1 |
S3 |
107.432 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
