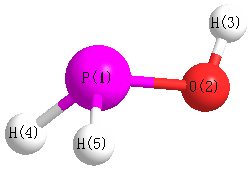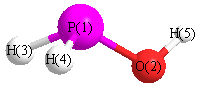Jump to
S1C2
Energy calculated at MP4/TZVP
| | hartrees |
|---|
| Energy at 0K | -417.741990 |
| Energy at 298.15K | -417.746117 |
| HF Energy | -417.374801 |
| Nuclear repulsion energy | 60.967826 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4/TZVP
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3844 |
3701 |
|
|
|
|
| 2 |
A' |
2388 |
2299 |
|
|
|
|
| 3 |
A' |
1172 |
1128 |
|
|
|
|
| 4 |
A' |
1110 |
1069 |
|
|
|
|
| 5 |
A' |
922 |
888 |
|
|
|
|
| 6 |
A' |
792 |
762 |
|
|
|
|
| 7 |
A" |
2390 |
2301 |
|
|
|
|
| 8 |
A" |
929 |
894 |
|
|
|
|
| 9 |
A" |
424 |
408 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 6984.2 cm
-1
Scaled (by 0.9629) Zero Point Vibrational Energy (zpe) 6725.1 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at MP4/TZVP
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| P1 |
-0.107 |
-0.578 |
0.000 |
| O2 |
-0.107 |
1.108 |
0.000 |
| H3 |
0.785 |
1.472 |
0.000 |
| H4 |
0.843 |
-0.836 |
1.031 |
| H5 |
0.843 |
-0.836 |
-1.031 |
Atom - Atom Distances (Å)
| |
P1 |
O2 |
H3 |
H4 |
H5 |
| P1 | | 1.6853 | 2.2356 | 1.4256 | 1.4256 |
O2 | 1.6853 | | 0.9638 | 2.3964 | 2.3964 | H3 | 2.2356 | 0.9638 | | 2.5284 | 2.5284 | H4 | 1.4256 | 2.3964 | 2.5284 | | 2.0614 | H5 | 1.4256 | 2.3964 | 2.5284 | 2.0614 | |
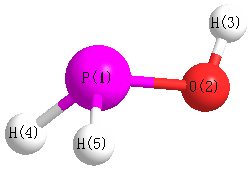 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| P1 |
O2 |
H3 |
112.227 |
|
O2 |
P1 |
H4 |
100.434 |
| O2 |
P1 |
H5 |
100.434 |
|
H4 |
P1 |
H5 |
92.603 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at MP4/TZVP
| | hartrees |
|---|
| Energy at 0K | -417.742525 |
| Energy at 298.15K | -417.746484 |
| HF Energy | -417.374293 |
| Nuclear repulsion energy | 60.870752 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4/TZVP
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3863 |
3720 |
|
|
|
|
| 2 |
A' |
2435 |
2344 |
|
|
|
|
| 3 |
A' |
1175 |
1132 |
|
|
|
|
| 4 |
A' |
1144 |
1101 |
|
|
|
|
| 5 |
A' |
916 |
882 |
|
|
|
|
| 6 |
A' |
781 |
752 |
|
|
|
|
| 7 |
A" |
2431 |
2341 |
|
|
|
|
| 8 |
A" |
941 |
906 |
|
|
|
|
| 9 |
A" |
269 |
259 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 6977.1 cm
-1
Scaled (by 0.9629) Zero Point Vibrational Energy (zpe) 6718.2 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at MP4/TZVP
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| P1 |
0.038 |
-0.583 |
0.000 |
| O2 |
0.038 |
1.113 |
0.000 |
| H3 |
0.958 |
1.397 |
0.000 |
| H4 |
-0.917 |
-0.779 |
1.031 |
| H5 |
-0.917 |
-0.779 |
-1.031 |
Atom - Atom Distances (Å)
| |
P1 |
O2 |
H3 |
H4 |
H5 |
| P1 | | 1.6955 | 2.1832 | 1.4187 | 1.4187 |
O2 | 1.6955 | | 0.9629 | 2.3561 | 2.3561 | H3 | 2.1832 | 0.9629 | | 3.0515 | 3.0515 | H4 | 1.4187 | 2.3561 | 3.0515 | | 2.0614 | H5 | 1.4187 | 2.3561 | 3.0515 | 2.0614 | |
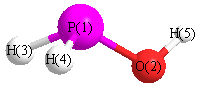 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| P1 |
O2 |
H3 |
107.179 |
|
O2 |
P1 |
H4 |
97.930 |
| O2 |
P1 |
H5 |
97.930 |
|
H4 |
P1 |
H5 |
93.184 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability