Jump to
S1C2
Energy calculated at MP4=FULL/6-31G*
| | hartrees |
|---|
| Energy at 0K | -341.515029 |
| Energy at 298.15K | |
| HF Energy | -340.521614 |
| Nuclear repulsion energy | 243.683502 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4=FULL/6-31G*
Geometric Data calculated at MP4=FULL/6-31G*
Point Group is C2v
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at MP4=FULL/6-31G*
| | hartrees |
|---|
| Energy at 0K | -341.516553 |
| Energy at 298.15K | -341.522944 |
| HF Energy | -340.521790 |
| Nuclear repulsion energy | 244.621199 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4=FULL/6-31G*
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
3141 |
3020 |
|
|
|
|
| 2 |
A |
3061 |
2943 |
|
|
|
|
| 3 |
A |
1911 |
1838 |
|
|
|
|
| 4 |
A |
1576 |
1515 |
|
|
|
|
| 5 |
A |
1425 |
1370 |
|
|
|
|
| 6 |
A |
1273 |
1224 |
|
|
|
|
| 7 |
A |
1213 |
1166 |
|
|
|
|
| 8 |
A |
1165 |
1120 |
|
|
|
|
| 9 |
A |
976 |
939 |
|
|
|
|
| 10 |
A |
808 |
777 |
|
|
|
|
| 11 |
A |
712 |
684 |
|
|
|
|
| 12 |
A |
173 |
166 |
|
|
|
|
| 13 |
B |
3151 |
3030 |
|
|
|
|
| 14 |
B |
3065 |
2947 |
|
|
|
|
| 15 |
B |
1568 |
1508 |
|
|
|
|
| 16 |
B |
1416 |
1362 |
|
|
|
|
| 17 |
B |
1281 |
1232 |
|
|
|
|
| 18 |
B |
1172 |
1127 |
|
|
|
|
| 19 |
B |
1042 |
1002 |
|
|
|
|
| 20 |
B |
919 |
884 |
|
|
|
|
| 21 |
B |
749 |
720 |
|
|
|
|
| 22 |
B |
674 |
648 |
|
|
|
|
| 23 |
B |
495 |
476 |
|
|
|
|
| 24 |
B |
150 |
145 |
|
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 16557.0 cm
-1
Scaled (by 0.9616) Zero Point Vibrational Energy (zpe) 15921.2 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at MP4=FULL/6-31G*
Point Group is C2
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| C1 |
0.000 |
0.000 |
0.864 |
| O2 |
0.000 |
0.000 |
2.062 |
| O3 |
0.000 |
1.139 |
0.042 |
| O4 |
0.000 |
-1.139 |
0.042 |
| C5 |
0.229 |
0.731 |
-1.283 |
| C6 |
-0.229 |
-0.731 |
-1.283 |
| H7 |
-0.349 |
1.369 |
-1.959 |
| H8 |
1.299 |
0.823 |
-1.519 |
| H9 |
0.349 |
-1.369 |
-1.959 |
| H10 |
-1.299 |
-0.823 |
-1.519 |
Atom - Atom Distances (Å)
| |
C1 |
O2 |
O3 |
O4 |
C5 |
C6 |
H7 |
H8 |
H9 |
H10 |
| C1 | | 1.1987 | 1.4047 | 1.4047 | 2.2790 | 2.2790 | 3.1564 | 2.8361 | 3.1564 | 2.8361 |
O2 | 1.1987 | | 2.3196 | 2.3196 | 3.4316 | 3.4316 | 4.2622 | 3.8978 | 4.2622 | 3.8978 | O3 | 1.4047 | 2.3196 | | 2.2784 | 1.4047 | 2.3031 | 2.0438 | 2.0555 | 3.2275 | 2.8238 | O4 | 1.4047 | 2.3196 | 2.2784 | | 2.3031 | 1.4047 | 3.2275 | 2.8238 | 2.0438 | 2.0555 | C5 | 2.2790 | 3.4316 | 1.4047 | 2.3031 | | 1.5320 | 1.0949 | 1.0999 | 2.2097 | 2.1921 | C6 | 2.2790 | 3.4316 | 2.3031 | 1.4047 | 1.5320 | | 2.2097 | 2.1921 | 1.0949 | 1.0999 | H7 | 3.1564 | 4.2622 | 2.0438 | 3.2275 | 1.0949 | 2.2097 | | 1.7913 | 2.8262 | 2.4292 | H8 | 2.8361 | 3.8978 | 2.0555 | 2.8238 | 1.0999 | 2.1921 | 1.7913 | | 2.4292 | 3.0756 | H9 | 3.1564 | 4.2622 | 3.2275 | 2.0438 | 2.2097 | 1.0949 | 2.8262 | 2.4292 | | 1.7913 | H10 | 2.8361 | 3.8978 | 2.8238 | 2.0555 | 2.1921 | 1.0999 | 2.4292 | 3.0756 | 1.7913 | |
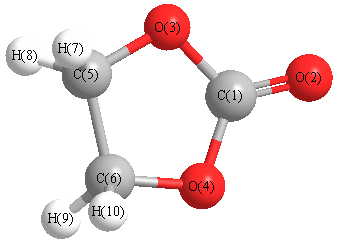 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
O3 |
C5 |
108.421 |
|
C1 |
O4 |
C6 |
108.421 |
| O2 |
C1 |
O3 |
125.810 |
|
O2 |
C1 |
O4 |
125.810 |
| O3 |
C1 |
O4 |
108.380 |
|
O3 |
C5 |
C6 |
103.212 |
| O3 |
C5 |
H7 |
109.069 |
|
O3 |
C5 |
H8 |
109.708 |
| O4 |
C6 |
C5 |
103.212 |
|
O4 |
C6 |
H9 |
109.069 |
| O4 |
C6 |
H10 |
109.708 |
|
C5 |
C6 |
H9 |
113.486 |
| C5 |
C6 |
H10 |
111.744 |
|
C6 |
C5 |
H7 |
113.486 |
| C6 |
C5 |
H8 |
111.744 |
|
H7 |
C5 |
H8 |
109.409 |
| H9 |
C6 |
H10 |
109.409 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
