All results from a given calculation for CHF2CH2F (Ethane, 1,1,2-trifluoro)
using model chemistry: MP4=FULL/Def2TZVPP
19 10 17 12 22
States and conformations
| State |
Conformation |
minimum conformation |
conformer description |
state description |
| 1 |
1 |
yes |
C1 |
1A |
Energy calculated at MP4=FULL/Def2TZVPP
| | hartrees |
|---|
| Energy at 0K | -377.192095 |
| Energy at 298.15K | |
| HF Energy | -375.956531 |
| Nuclear repulsion energy | 190.861104 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4=FULL/Def2TZVPP
Geometric Data calculated at MP4=FULL/Def2TZVPP
Point Group is C1
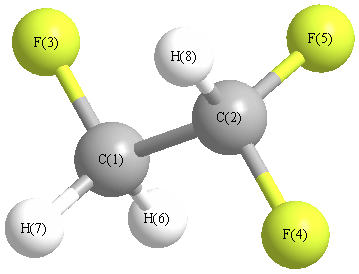
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| C1 |
-0.771 |
-0.593 |
-0.283 |
| C2 |
0.466 |
0.020 |
0.330 |
| F3 |
-1.880 |
0.109 |
0.150 |
| F4 |
1.533 |
-0.758 |
-0.008 |
| F5 |
0.677 |
1.260 |
-0.182 |
| H6 |
-0.706 |
-0.531 |
-1.367 |
| H7 |
-0.859 |
-1.630 |
0.036 |
| H8 |
0.417 |
0.102 |
1.413 |
Atom - Atom Distances (Å)
| |
C1 |
C2 |
F3 |
F4 |
F5 |
H6 |
H7 |
H8 |
| C1 | | 1.5105 | 1.3817 | 2.3265 | 2.3541 | 1.0879 | 1.0880 | 2.1844 |
C2 | 1.5105 | | 2.3542 | 1.3631 | 1.3578 | 2.1347 | 2.1368 | 1.0877 | F3 | 1.3817 | 2.3542 | | 3.5248 | 2.8233 | 2.0215 | 2.0192 | 2.6214 | F4 | 2.3265 | 1.3631 | 3.5248 | | 2.1987 | 2.6294 | 2.5474 | 2.0013 | F5 | 2.3541 | 1.3578 | 2.8233 | 2.1987 | | 2.5547 | 3.2802 | 1.9881 | H6 | 1.0879 | 2.1347 | 2.0215 | 2.6294 | 2.5547 | | 1.7883 | 3.0647 | H7 | 1.0880 | 2.1368 | 2.0192 | 2.5474 | 3.2802 | 1.7883 | | 2.5549 | H8 | 2.1844 | 1.0877 | 2.6214 | 2.0013 | 1.9881 | 3.0647 | 2.5549 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C2 |
F4 |
108.005 |
|
C1 |
C2 |
F5 |
110.201 |
| C1 |
C2 |
H8 |
113.437 |
|
C2 |
C1 |
F3 |
108.894 |
| C2 |
C1 |
H6 |
109.399 |
|
C2 |
C1 |
H7 |
109.567 |
| F3 |
C1 |
H6 |
109.300 |
|
F3 |
C1 |
H7 |
109.111 |
| F4 |
C2 |
F5 |
107.812 |
|
F4 |
C2 |
H8 |
108.974 |
| F5 |
C2 |
H8 |
108.263 |
|
H6 |
C1 |
H7 |
110.543 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
