All results from a given calculation for C2H5I (Ethyl iodide)
using model chemistry: MP4=FULL/Def2TZVPP
19 10 17 12 22
States and conformations
| State |
Conformation |
minimum conformation |
conformer description |
state description |
| 1 |
1 |
yes |
CS |
1A' |
Energy calculated at MP4=FULL/Def2TZVPP
| | hartrees |
|---|
| Energy at 0K | -376.349383 |
| Energy at 298.15K | |
| HF Energy | -375.339479 |
| Nuclear repulsion energy | 121.659521 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at MP4=FULL/Def2TZVPP
Geometric Data calculated at MP4=FULL/Def2TZVPP
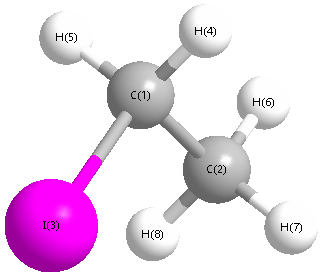
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| C1 |
0.610 |
-1.417 |
0.000 |
| C2 |
-0.581 |
-2.356 |
0.000 |
| I3 |
0.000 |
0.632 |
0.000 |
| H4 |
1.227 |
-1.532 |
0.885 |
| H5 |
1.227 |
-1.532 |
-0.885 |
| H6 |
-0.231 |
-3.390 |
0.000 |
| H7 |
-1.200 |
-2.202 |
0.882 |
| H8 |
-1.200 |
-2.202 |
-0.882 |
Atom - Atom Distances (Å)
| |
C1 |
C2 |
I3 |
H4 |
H5 |
H6 |
H7 |
H8 |
| C1 | | 1.5164 | 2.1378 | 1.0851 | 1.0851 | 2.1447 | 2.1610 | 2.1610 |
C2 | 1.5164 | | 3.0435 | 2.1753 | 2.1753 | 1.0918 | 1.0882 | 1.0882 | I3 | 2.1378 | 3.0435 | | 2.6402 | 2.6402 | 4.0284 | 3.2012 | 3.2012 | H4 | 1.0851 | 2.1753 | 2.6402 | | 1.7703 | 2.5225 | 2.5182 | 3.0761 | H5 | 1.0851 | 2.1753 | 2.6402 | 1.7703 | | 2.5225 | 3.0761 | 2.5182 | H6 | 2.1447 | 1.0918 | 4.0284 | 2.5225 | 2.5225 | | 1.7685 | 1.7685 | H7 | 2.1610 | 1.0882 | 3.2012 | 2.5182 | 3.0761 | 1.7685 | | 1.7632 | H8 | 2.1610 | 1.0882 | 3.2012 | 3.0761 | 2.5182 | 1.7685 | 1.7632 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C2 |
H6 |
109.552 |
|
C1 |
C2 |
H7 |
111.066 |
| C1 |
C2 |
H8 |
111.066 |
|
C2 |
C1 |
I3 |
111.663 |
| C2 |
C1 |
H4 |
112.414 |
|
C2 |
C1 |
H5 |
112.414 |
| I3 |
C1 |
H4 |
105.283 |
|
I3 |
C1 |
H5 |
105.283 |
| H4 |
C1 |
H5 |
109.308 |
|
H6 |
C2 |
H7 |
108.430 |
| H6 |
C2 |
H8 |
108.430 |
|
H7 |
C2 |
H8 |
108.216 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
