All results from a given calculation for C6H13N (cyclohexanamine)
using model chemistry: CCD/6-31+G**
19 10 17 12 22
States and conformations
| State |
Conformation |
minimum conformation |
conformer description |
state description |
| 1 |
1 |
yes |
C1 |
1A |
Energy calculated at CCD/6-31+G**
| | hartrees |
|---|
| Energy at 0K | -290.371607 |
| Energy at 298.15K | |
| HF Energy | -289.258331 |
| Nuclear repulsion energy | 330.138948 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCD/6-31+G**
Geometric Data calculated at CCD/6-31+G**
Point Group is C1
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| C1 |
1.879 |
0.012 |
0.291 |
| C2 |
1.180 |
-1.253 |
-0.217 |
| C3 |
-0.305 |
-1.265 |
0.158 |
| C4 |
-1.029 |
-0.010 |
-0.332 |
| C5 |
-0.320 |
1.253 |
0.179 |
| C6 |
1.164 |
1.272 |
-0.205 |
| N7 |
-2.448 |
-0.099 |
0.034 |
| H8 |
2.925 |
0.020 |
-0.026 |
| H9 |
1.881 |
0.007 |
1.387 |
| H10 |
1.276 |
-1.304 |
-1.307 |
| H11 |
1.672 |
-2.144 |
0.182 |
| H12 |
-0.805 |
-2.146 |
-0.252 |
| H13 |
-0.404 |
-1.321 |
1.250 |
| H14 |
-0.992 |
-0.003 |
-1.428 |
| H15 |
-0.416 |
1.288 |
1.271 |
| H16 |
-0.827 |
2.141 |
-0.211 |
| H17 |
1.645 |
2.167 |
0.199 |
| H18 |
1.254 |
1.332 |
-1.296 |
| H19 |
-2.951 |
0.718 |
-0.296 |
| H20 |
-2.543 |
-0.114 |
1.045 |
Atom - Atom Distances (Å)
| |
C1 |
C2 |
C3 |
C4 |
C5 |
C6 |
N7 |
H8 |
H9 |
H10 |
H11 |
H12 |
H13 |
H14 |
H15 |
H16 |
H17 |
H18 |
H19 |
H20 |
| C1 | | 1.5319 | 2.5339 | 2.9744 | 2.5275 | 1.5317 | 4.3358 | 1.0931 | 1.0960 | 2.1564 | 2.1688 | 3.4859 | 2.8123 | 3.3466 | 2.8029 | 3.4800 | 2.1700 | 2.1570 | 4.9163 | 4.4880 |
C2 | 1.5319 | | 1.5322 | 2.5382 | 2.9476 | 2.5258 | 3.8154 | 2.1682 | 2.1573 | 1.0959 | 1.0929 | 2.1766 | 2.1600 | 2.7841 | 3.3494 | 3.9436 | 3.4768 | 2.8028 | 4.5780 | 4.0935 | C3 | 2.5339 | 1.5322 | | 1.5296 | 2.5184 | 2.9548 | 2.4422 | 3.4815 | 2.8131 | 2.1562 | 2.1642 | 1.0918 | 1.0978 | 2.1401 | 2.7872 | 3.4656 | 3.9480 | 3.3604 | 3.3372 | 2.6687 | C4 | 2.9744 | 2.5382 | 1.5296 | | 1.5357 | 2.5439 | 1.4674 | 3.9661 | 3.3804 | 2.8182 | 3.4811 | 2.1491 | 2.1473 | 1.0970 | 2.1511 | 2.1634 | 3.4888 | 2.8187 | 2.0549 | 2.0489 | C5 | 2.5275 | 2.9476 | 2.5184 | 1.5357 | | 1.5328 | 2.5254 | 3.4771 | 2.8030 | 3.3605 | 3.9382 | 3.4599 | 2.7895 | 2.1471 | 1.0974 | 1.0945 | 2.1671 | 2.1579 | 2.7265 | 2.7501 | C6 | 1.5317 | 2.5258 | 2.9548 | 2.5439 | 1.5328 | | 3.8708 | 2.1681 | 2.1562 | 2.8045 | 3.4759 | 3.9447 | 3.3619 | 2.7878 | 2.1626 | 2.1727 | 1.0930 | 1.0960 | 4.1530 | 4.1508 | N7 | 4.3358 | 3.8154 | 2.4422 | 1.4674 | 2.5254 | 3.8708 | | 5.3743 | 4.5369 | 4.1373 | 4.6019 | 2.6399 | 2.6734 | 2.0650 | 2.7532 | 2.7754 | 4.6811 | 4.1857 | 1.0149 | 1.0158 | H8 | 1.0931 | 2.1682 | 3.4815 | 3.9661 | 3.4771 | 2.1681 | 5.3743 | | 1.7571 | 2.4722 | 2.5094 | 4.3186 | 3.8093 | 4.1604 | 3.8019 | 4.3142 | 2.5098 | 2.4749 | 5.9231 | 5.5739 | H9 | 1.0960 | 2.1573 | 2.8131 | 3.3804 | 2.8030 | 2.1562 | 4.5369 | 1.7571 | | 3.0572 | 2.4754 | 3.8128 | 2.6474 | 4.0228 | 2.6327 | 3.8005 | 2.4763 | 3.0572 | 5.1661 | 4.4396 | H10 | 2.1564 | 1.0959 | 2.1562 | 2.8182 | 3.3605 | 2.8045 | 4.1373 | 2.4722 | 3.0572 | | 1.7551 | 2.4803 | 3.0597 | 2.6179 | 4.0284 | 4.1825 | 3.8016 | 2.6366 | 4.7934 | 4.6410 | H11 | 2.1688 | 1.0929 | 2.1642 | 3.4811 | 3.9382 | 3.4759 | 4.6019 | 2.5094 | 2.4754 | 1.7551 | | 2.5144 | 2.4757 | 3.7784 | 4.1624 | 4.9765 | 4.3116 | 3.8007 | 5.4582 | 4.7580 | H12 | 3.4859 | 2.1766 | 1.0918 | 2.1491 | 3.4599 | 3.9447 | 2.6399 | 4.3186 | 3.8128 | 2.4803 | 2.5144 | | 1.7591 | 2.4516 | 3.7757 | 4.2867 | 4.9803 | 4.1743 | 3.5788 | 2.9722 | H13 | 2.8123 | 2.1600 | 1.0978 | 2.1473 | 2.7895 | 3.3619 | 2.6734 | 3.8093 | 2.6474 | 3.0597 | 2.4757 | 1.7591 | | 3.0419 | 2.6088 | 3.7814 | 4.1800 | 4.0337 | 3.6101 | 2.4649 | H14 | 3.3466 | 2.7841 | 2.1401 | 1.0970 | 2.1471 | 2.7878 | 2.0650 | 4.1604 | 4.0228 | 2.6179 | 3.7784 | 2.4516 | 3.0419 | | 3.0465 | 2.4703 | 3.7827 | 2.6166 | 2.3739 | 2.9212 | H15 | 2.8029 | 3.3494 | 2.7872 | 2.1511 | 1.0974 | 2.1626 | 2.7532 | 3.8019 | 2.6327 | 4.0284 | 4.1624 | 3.7757 | 2.6088 | 3.0465 | | 1.7592 | 2.4845 | 3.0628 | 3.0338 | 2.5571 | H16 | 3.4800 | 3.9436 | 3.4656 | 2.1634 | 1.0945 | 2.1727 | 2.7754 | 4.3142 | 3.8005 | 4.1825 | 4.9765 | 4.2867 | 3.7814 | 2.4703 | 1.7592 | | 2.5063 | 2.4824 | 2.5573 | 3.0993 | H17 | 2.1700 | 3.4768 | 3.9480 | 3.4888 | 2.1671 | 1.0930 | 4.6811 | 2.5098 | 2.4763 | 3.8016 | 4.3116 | 4.9803 | 4.1800 | 3.7827 | 2.4845 | 2.5063 | | 1.7556 | 4.8441 | 4.8436 | H18 | 2.1570 | 2.8028 | 3.3604 | 2.8187 | 2.1579 | 1.0960 | 4.1857 | 2.4749 | 3.0572 | 2.6366 | 3.8007 | 4.1743 | 4.0337 | 2.6166 | 3.0628 | 2.4824 | 1.7556 | | 4.3654 | 4.6894 | H19 | 4.9163 | 4.5780 | 3.3372 | 2.0549 | 2.7265 | 4.1530 | 1.0149 | 5.9231 | 5.1661 | 4.7934 | 5.4582 | 3.5788 | 3.6101 | 2.3739 | 3.0338 | 2.5573 | 4.8441 | 4.3654 | | 1.6300 | H20 | 4.4880 | 4.0935 | 2.6687 | 2.0489 | 2.7501 | 4.1508 | 1.0158 | 5.5739 | 4.4396 | 4.6410 | 4.7580 | 2.9722 | 2.4649 | 2.9212 | 2.5571 | 3.0993 | 4.8436 | 4.6894 | 1.6300 | |
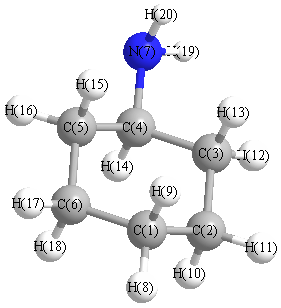 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C2 |
C3 |
111.570 |
|
C1 |
C2 |
H10 |
109.158 |
| C1 |
C2 |
H11 |
110.314 |
|
C1 |
C6 |
C5 |
111.126 |
| C1 |
C6 |
H17 |
110.412 |
|
C1 |
C6 |
H18 |
109.214 |
| C2 |
C1 |
C6 |
111.062 |
|
C2 |
C1 |
H8 |
110.258 |
| C2 |
C1 |
H9 |
109.227 |
|
C2 |
C3 |
C4 |
111.985 |
| C2 |
C3 |
H12 |
110.975 |
|
C2 |
C3 |
H13 |
109.317 |
| C3 |
C2 |
H10 |
109.125 |
|
C3 |
C2 |
H11 |
109.930 |
| C3 |
C4 |
C5 |
110.485 |
|
C3 |
C4 |
N7 |
109.133 |
| C3 |
C4 |
H14 |
107.995 |
|
C4 |
C3 |
H12 |
108.991 |
| C4 |
C3 |
H13 |
108.506 |
|
C4 |
C5 |
C6 |
111.999 |
| C4 |
C5 |
H15 |
108.415 |
|
C4 |
C5 |
H16 |
109.533 |
| C4 |
N7 |
H19 |
110.426 |
|
C4 |
N7 |
H20 |
109.867 |
| C5 |
C4 |
N7 |
114.457 |
|
C5 |
C4 |
H14 |
108.128 |
| C5 |
C6 |
H17 |
110.111 |
|
C5 |
C6 |
H18 |
109.218 |
| C6 |
C1 |
H8 |
110.262 |
|
C6 |
C1 |
H9 |
109.153 |
| C6 |
C5 |
H15 |
109.506 |
|
C6 |
C5 |
H16 |
110.470 |
| N7 |
C4 |
H14 |
106.377 |
|
H8 |
C1 |
H9 |
106.771 |
| H10 |
C2 |
H11 |
106.607 |
|
H12 |
C3 |
H13 |
106.907 |
| H15 |
C5 |
H16 |
106.758 |
|
H17 |
C6 |
H18 |
106.644 |
| H19 |
N7 |
H20 |
106.777 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
