Jump to
S1C2
Energy calculated at CCD/cc-pVTZ
| | hartrees |
|---|
| Energy at 0K | -1194.383175 |
| Energy at 298.15K | -1194.385565 |
| HF Energy | -1193.812294 |
| Nuclear repulsion energy | 195.499603 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCD/cc-pVTZ
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A |
2709 |
2529 |
0.26 |
|
|
|
| 2 |
A |
900 |
841 |
0.02 |
|
|
|
| 3 |
A |
506 |
472 |
0.34 |
|
|
|
| 4 |
A |
305 |
284 |
19.80 |
|
|
|
| 5 |
A |
210 |
196 |
0.01 |
|
|
|
| 6 |
B |
2708 |
2528 |
1.12 |
|
|
|
| 7 |
B |
890 |
831 |
7.98 |
|
|
|
| 8 |
B |
512 |
478 |
15.90 |
|
|
|
| 9 |
B |
328 |
307 |
14.50 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4533.6 cm
-1
Scaled (by 0.9337) Zero Point Vibrational Energy (zpe) 4233.0 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CCD/cc-pVTZ
Point Group is C2
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| S1 |
0.000 |
0.000 |
0.850 |
| S2 |
0.000 |
1.659 |
-0.390 |
| S3 |
0.000 |
-1.659 |
-0.390 |
| H4 |
-1.326 |
1.749 |
-0.570 |
| H5 |
1.326 |
-1.749 |
-0.570 |
Atom - Atom Distances (Å)
| |
S1 |
S2 |
S3 |
H4 |
H5 |
| S1 | | 2.0711 | 2.0711 | 2.6146 | 2.6146 |
S2 | 2.0711 | | 3.3176 | 1.3415 | 3.6615 | S3 | 2.0711 | 3.3176 | | 3.6615 | 1.3415 | H4 | 2.6146 | 1.3415 | 3.6615 | | 4.3904 | H5 | 2.6146 | 3.6615 | 1.3415 | 4.3904 | |
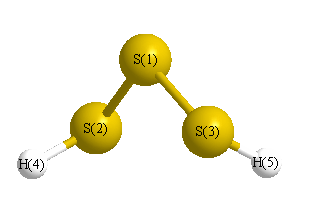 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| S1 |
S2 |
H4 |
97.728 |
|
S1 |
S3 |
H5 |
97.728 |
| S2 |
S1 |
S3 |
106.441 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at CCD/cc-pVTZ
| | hartrees |
|---|
| Energy at 0K | -1194.382991 |
| Energy at 298.15K | -1194.385393 |
| HF Energy | -1193.812023 |
| Nuclear repulsion energy | 195.537495 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCD/cc-pVTZ
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
2703 |
2524 |
2.92 |
|
|
|
| 2 |
A' |
903 |
843 |
3.80 |
|
|
|
| 3 |
A' |
506 |
472 |
0.34 |
|
|
|
| 4 |
A' |
329 |
308 |
15.27 |
|
|
|
| 5 |
A' |
211 |
197 |
0.05 |
|
|
|
| 6 |
A" |
2705 |
2526 |
0.22 |
|
|
|
| 7 |
A" |
892 |
833 |
5.16 |
|
|
|
| 8 |
A" |
513 |
479 |
17.10 |
|
|
|
| 9 |
A" |
312 |
291 |
9.09 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 4536.8 cm
-1
Scaled (by 0.9337) Zero Point Vibrational Energy (zpe) 4236.0 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at CCD/cc-pVTZ
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| S1 |
-0.053 |
0.849 |
0.000 |
| S2 |
-0.053 |
-0.392 |
1.658 |
| S3 |
-0.053 |
-0.392 |
-1.658 |
| H4 |
1.277 |
-0.515 |
1.789 |
| H5 |
1.277 |
-0.515 |
-1.789 |
Atom - Atom Distances (Å)
| |
S1 |
S2 |
S3 |
H4 |
H5 |
| S1 | | 2.0707 | 2.0707 | 2.6130 | 2.6130 |
S2 | 2.0707 | | 3.3152 | 1.3418 | 3.6961 | S3 | 2.0707 | 3.3152 | | 3.6961 | 1.3418 | H4 | 2.6130 | 1.3418 | 3.6961 | | 3.5776 | H5 | 2.6130 | 3.6961 | 1.3418 | 3.5776 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| S1 |
S2 |
H4 |
97.650 |
|
S1 |
S3 |
H5 |
97.650 |
| S2 |
S1 |
S3 |
106.357 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
