Jump to
S1C2
Vibrational Frequencies calculated at CCD/cc-pVTZ
Geometric Data calculated at CCD/cc-pVTZ
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at CCD/cc-pVTZ
| | hartrees |
|---|
| Energy at 0K | -235.348297 |
| Energy at 298.15K | |
| HF Energy | -234.249355 |
| Nuclear repulsion energy | 250.375403 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at CCD/cc-pVTZ
Geometric Data calculated at CCD/cc-pVTZ
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| C1 |
0.274 |
0.098 |
1.078 |
| C2 |
0.274 |
0.098 |
-1.078 |
| C3 |
-0.669 |
0.688 |
0.000 |
| C4 |
0.873 |
-0.842 |
0.000 |
| C5 |
-0.920 |
2.183 |
0.000 |
| C6 |
0.274 |
-2.240 |
0.000 |
| H7 |
0.996 |
0.838 |
1.426 |
| H8 |
-0.188 |
-0.375 |
1.945 |
| H9 |
0.996 |
0.838 |
-1.426 |
| H10 |
-0.188 |
-0.375 |
-1.945 |
| H11 |
-1.621 |
0.155 |
0.000 |
| H12 |
1.960 |
-0.908 |
0.000 |
| H13 |
0.025 |
2.731 |
0.000 |
| H14 |
-0.817 |
-2.199 |
0.000 |
| H15 |
-1.485 |
2.488 |
-0.883 |
| H16 |
-1.485 |
2.488 |
0.883 |
| H17 |
0.587 |
-2.798 |
-0.884 |
| H18 |
0.587 |
-2.798 |
0.884 |
Atom - Atom Distances (Å)
| |
C1 |
C2 |
C3 |
C4 |
C5 |
C6 |
H7 |
H8 |
H9 |
H10 |
H11 |
H12 |
H13 |
H14 |
H15 |
H16 |
H17 |
H18 |
| C1 | | 2.1567 | 1.5490 | 1.5510 | 2.6333 | 2.5745 | 1.0908 | 1.0901 | 2.7090 | 3.0949 | 2.1809 | 2.2401 | 2.8558 | 2.7622 | 3.5564 | 2.9733 | 3.5120 | 2.9194 |
C2 | 2.1567 | | 1.5490 | 1.5510 | 2.6333 | 2.5745 | 2.7090 | 3.0949 | 1.0908 | 1.0901 | 2.1809 | 2.2401 | 2.8558 | 2.7622 | 2.9733 | 3.5564 | 2.9194 | 3.5120 | C3 | 1.5490 | 1.5490 | | 2.1719 | 1.5163 | 3.0754 | 2.1971 | 2.2680 | 2.1971 | 2.2680 | 1.0908 | 3.0753 | 2.1577 | 2.8902 | 2.1644 | 2.1644 | 3.8089 | 3.8089 | C4 | 1.5510 | 1.5510 | 2.1719 | | 3.5163 | 1.5202 | 2.2071 | 2.2642 | 2.2071 | 2.2642 | 2.6855 | 1.0894 | 3.6722 | 2.1671 | 4.1743 | 4.1743 | 2.1649 | 2.1649 | C5 | 2.6333 | 2.6333 | 1.5163 | 3.5163 | | 4.5810 | 2.7406 | 3.2958 | 2.7406 | 3.2958 | 2.1460 | 4.2245 | 1.0917 | 4.3831 | 1.0914 | 1.0914 | 5.2784 | 5.2784 | C6 | 2.5745 | 2.5745 | 3.0754 | 1.5202 | 4.5810 | | 3.4679 | 2.7339 | 3.4679 | 2.7339 | 3.0536 | 2.1485 | 4.9767 | 1.0920 | 5.1205 | 5.1205 | 1.0911 | 1.0911 | H7 | 1.0908 | 2.7090 | 2.1971 | 2.2071 | 2.7406 | 3.4679 | | 1.7729 | 2.8510 | 3.7729 | 3.0574 | 2.4516 | 2.5608 | 3.8136 | 3.7686 | 3.0281 | 4.3266 | 3.6989 | H8 | 1.0901 | 3.0949 | 2.2680 | 2.2642 | 3.2958 | 2.7339 | 1.7729 | | 3.7729 | 3.8903 | 2.4734 | 2.9464 | 3.6707 | 2.7398 | 4.2276 | 3.3174 | 3.8044 | 2.7565 | H9 | 2.7090 | 1.0908 | 2.1971 | 2.2071 | 2.7406 | 3.4679 | 2.8510 | 3.7729 | | 1.7729 | 3.0574 | 2.4516 | 2.5608 | 3.8136 | 3.0281 | 3.7686 | 3.6989 | 4.3266 | H10 | 3.0949 | 1.0901 | 2.2680 | 2.2642 | 3.2958 | 2.7339 | 3.7729 | 3.8903 | 1.7729 | | 2.4734 | 2.9464 | 3.6707 | 2.7398 | 3.3174 | 4.2276 | 2.7565 | 3.8044 | H11 | 2.1809 | 2.1809 | 1.0908 | 2.6855 | 2.1460 | 3.0536 | 3.0574 | 2.4734 | 3.0574 | 2.4734 | | 3.7353 | 3.0567 | 2.4871 | 2.4980 | 2.4980 | 3.7915 | 3.7915 | H12 | 2.2401 | 2.2401 | 3.0753 | 1.0894 | 4.2245 | 2.1485 | 2.4516 | 2.9464 | 2.4516 | 2.9464 | 3.7353 | | 4.1214 | 3.0626 | 4.9168 | 4.9168 | 2.4974 | 2.4974 | H13 | 2.8558 | 2.8558 | 2.1577 | 3.6722 | 1.0917 | 4.9767 | 2.5608 | 3.6707 | 2.5608 | 3.6707 | 3.0567 | 4.1214 | | 5.0010 | 1.7653 | 1.7653 | 5.6270 | 5.6270 | H14 | 2.7622 | 2.7622 | 2.8902 | 2.1671 | 4.3831 | 1.0920 | 3.8136 | 2.7398 | 3.8136 | 2.7398 | 2.4871 | 3.0626 | 5.0010 | | 4.8154 | 4.8154 | 1.7642 | 1.7642 | H15 | 3.5564 | 2.9733 | 2.1644 | 4.1743 | 1.0914 | 5.1205 | 3.7686 | 4.2276 | 3.0281 | 3.3174 | 2.4980 | 4.9168 | 1.7653 | 4.8154 | | 1.7653 | 5.6772 | 5.9456 | H16 | 2.9733 | 3.5564 | 2.1644 | 4.1743 | 1.0914 | 5.1205 | 3.0281 | 3.3174 | 3.7686 | 4.2276 | 2.4980 | 4.9168 | 1.7653 | 4.8154 | 1.7653 | | 5.9456 | 5.6772 | H17 | 3.5120 | 2.9194 | 3.8089 | 2.1649 | 5.2784 | 1.0911 | 4.3266 | 3.8044 | 3.6989 | 2.7565 | 3.7915 | 2.4974 | 5.6270 | 1.7642 | 5.6772 | 5.9456 | | 1.7672 | H18 | 2.9194 | 3.5120 | 3.8089 | 2.1649 | 5.2784 | 1.0911 | 3.6989 | 2.7565 | 4.3266 | 3.8044 | 3.7915 | 2.4974 | 5.6270 | 1.7642 | 5.9456 | 5.6772 | 1.7672 | |
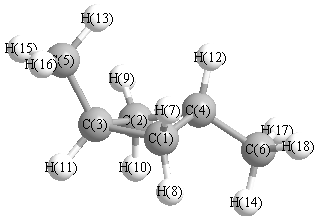 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C3 |
C2 |
88.240 |
|
C1 |
C3 |
C5 |
118.419 |
| C1 |
C3 |
H11 |
110.201 |
|
C1 |
C4 |
C2 |
88.096 |
| C1 |
C4 |
C6 |
113.910 |
|
C1 |
C4 |
H12 |
114.956 |
| C2 |
C3 |
C5 |
118.419 |
|
C2 |
C3 |
H11 |
110.201 |
| C2 |
C4 |
C6 |
113.910 |
|
C2 |
C4 |
H12 |
114.956 |
| C3 |
C1 |
C4 |
88.952 |
|
C3 |
C1 |
H7 |
111.495 |
| C3 |
C1 |
H8 |
117.441 |
|
C3 |
C2 |
C4 |
88.952 |
| C3 |
C2 |
H9 |
111.495 |
|
C3 |
C2 |
H10 |
117.441 |
| C3 |
C5 |
H13 |
110.601 |
|
C3 |
C5 |
H15 |
111.152 |
| C3 |
C5 |
H16 |
111.152 |
|
C4 |
C1 |
H7 |
112.150 |
| C4 |
C1 |
H8 |
116.954 |
|
C4 |
C2 |
H9 |
112.150 |
| C4 |
C2 |
H10 |
116.954 |
|
C4 |
C6 |
H14 |
111.047 |
| C4 |
C6 |
H17 |
110.928 |
|
C4 |
C6 |
H18 |
110.928 |
| C5 |
C3 |
H11 |
109.724 |
|
C6 |
C4 |
H12 |
109.731 |
| H7 |
C1 |
H8 |
108.772 |
|
H9 |
C2 |
H10 |
108.772 |
| H13 |
C5 |
H15 |
107.927 |
|
H13 |
C5 |
H16 |
107.927 |
| H14 |
C6 |
H17 |
107.822 |
|
H14 |
C6 |
H18 |
107.822 |
| H15 |
C5 |
H16 |
107.946 |
|
H17 |
C6 |
H18 |
108.158 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
