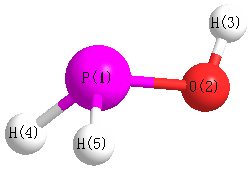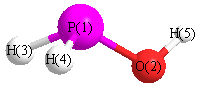Jump to
S1C2
Energy calculated at PBEPBEultrafine/6-31G*
| | hartrees |
|---|
| Energy at 0K | -418.095634 |
| Energy at 298.15K | -418.099705 |
| HF Energy | -418.095634 |
| Nuclear repulsion energy | 60.682833 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at PBEPBEultrafine/6-31G*
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3628 |
3568 |
25.64 |
|
|
|
| 2 |
A' |
2239 |
2202 |
155.90 |
|
|
|
| 3 |
A' |
1139 |
1120 |
6.84 |
|
|
|
| 4 |
A' |
1109 |
1091 |
67.78 |
|
|
|
| 5 |
A' |
882 |
867 |
45.11 |
|
|
|
| 6 |
A' |
769 |
756 |
118.90 |
|
|
|
| 7 |
A" |
2250 |
2213 |
202.62 |
|
|
|
| 8 |
A" |
860 |
846 |
16.48 |
|
|
|
| 9 |
A" |
424 |
417 |
114.57 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 6649.5 cm
-1
Scaled (by 0.9835) Zero Point Vibrational Energy (zpe) 6539.8 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at PBEPBEultrafine/6-31G*
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| P1 |
-0.110 |
-0.573 |
0.000 |
| O2 |
-0.110 |
1.109 |
0.000 |
| H3 |
0.795 |
1.483 |
0.000 |
| H4 |
0.862 |
-0.877 |
1.033 |
| H5 |
0.862 |
-0.877 |
-1.033 |
Atom - Atom Distances (Å)
| |
P1 |
O2 |
H3 |
H4 |
H5 |
| P1 | | 1.6825 | 2.2466 | 1.4503 | 1.4503 |
O2 | 1.6825 | | 0.9791 | 2.4406 | 2.4406 | H3 | 2.2466 | 0.9791 | | 2.5770 | 2.5770 | H4 | 1.4503 | 2.4406 | 2.5770 | | 2.0653 | H5 | 1.4503 | 2.4406 | 2.5770 | 2.0653 | |
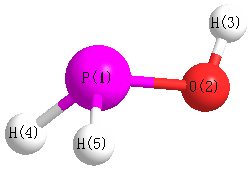 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| P1 |
O2 |
H3 |
112.445 |
|
O2 |
P1 |
H4 |
102.093 |
| O2 |
P1 |
H5 |
102.093 |
|
H4 |
P1 |
H5 |
90.800 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Charges from optimized geometry at PBEPBEultrafine/6-31G*
Charges (e)
| Number |
Element |
Mulliken |
CHELPG |
AIM |
ESP |
| 1 |
P |
0.261 |
|
|
|
| 2 |
O |
-0.652 |
|
|
|
| 3 |
H |
0.409 |
|
|
|
| 4 |
H |
-0.009 |
|
|
|
| 5 |
H |
-0.009 |
|
|
|
Electric dipole moments
Electric dipole components in Debye
(What's a Debye? See section
VII.A.3)
| |
x |
y |
z |
Total |
| |
2.321 |
0.121 |
0.000 |
2.324 |
| CHELPG |
|
|
|
|
| AIM |
|
|
|
|
| ESP |
|
|
|
|
Electric Quadrupole moment
Quadrupole components in D Å
| Primitive |
|---|
| | x | y | z |
|---|
| x |
-20.150 |
1.522 |
0.000 |
| y |
1.522 |
-19.461 |
0.000 |
| z |
0.000 |
0.000 |
-20.054 |
|
| Traceless |
|---|
| | x | y | z |
|---|
| x |
-0.392 |
1.522 |
0.000 |
| y |
1.522 |
0.641 |
0.000 |
| z |
0.000 |
0.000 |
-0.249 |
|
| Polar |
|---|
| 3z2-r2 | -0.498 |
|---|
| x2-y2 | -0.689 |
|---|
| xy | 1.522 |
|---|
| xz | 0.000 |
|---|
| yz | 0.000 |
|---|
|
Polarizabilities
Components of the polarizability tensor.
Units are
Å
3 (Angstrom cubed)
Change units.
| |
x |
y |
z |
| x |
3.378 |
-0.305 |
0.000 |
| y |
-0.305 |
3.831 |
0.000 |
| z |
0.000 |
0.000 |
3.540 |
<r2> (average value of r
2) Å
2
| <r2> |
35.460 |
| (<r2>)1/2 |
5.955 |
Jump to
S1C1
Energy calculated at PBEPBEultrafine/6-31G*
| | hartrees |
|---|
| Energy at 0K | -418.097317 |
| Energy at 298.15K | -418.101189 |
| HF Energy | -418.097317 |
| Nuclear repulsion energy | 60.567280 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at PBEPBEultrafine/6-31G*
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A' |
3675 |
3614 |
66.93 |
|
|
|
| 2 |
A' |
2300 |
2262 |
123.79 |
|
|
|
| 3 |
A' |
1156 |
1136 |
125.98 |
|
|
|
| 4 |
A' |
1135 |
1116 |
24.30 |
|
|
|
| 5 |
A' |
885 |
871 |
17.84 |
|
|
|
| 6 |
A' |
762 |
750 |
89.47 |
|
|
|
| 7 |
A" |
2304 |
2266 |
153.84 |
|
|
|
| 8 |
A" |
894 |
880 |
0.36 |
|
|
|
| 9 |
A" |
237 |
233 |
89.03 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 6673.9 cm
-1
Scaled (by 0.9835) Zero Point Vibrational Energy (zpe) 6563.8 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at PBEPBEultrafine/6-31G*
Point Group is Cs
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| P1 |
0.039 |
-0.581 |
0.000 |
| O2 |
0.039 |
1.116 |
0.000 |
| H3 |
0.978 |
1.388 |
0.000 |
| H4 |
-0.943 |
-0.801 |
1.031 |
| H5 |
-0.943 |
-0.801 |
-1.031 |
Atom - Atom Distances (Å)
| |
P1 |
O2 |
H3 |
H4 |
H5 |
| P1 | | 1.6963 | 2.1811 | 1.4412 | 1.4412 |
O2 | 1.6963 | | 0.9770 | 2.3879 | 2.3879 | H3 | 2.1811 | 0.9770 | | 3.0895 | 3.0895 | H4 | 1.4412 | 2.3879 | 3.0895 | | 2.0624 | H5 | 1.4412 | 2.3879 | 3.0895 | 2.0624 | |
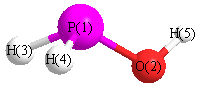 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| P1 |
O2 |
H3 |
106.205 |
|
O2 |
P1 |
H4 |
98.793 |
| O2 |
P1 |
H5 |
98.793 |
|
H4 |
P1 |
H5 |
91.375 |
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Charges from optimized geometry at PBEPBEultrafine/6-31G*
Charges (e)
| Number |
Element |
Mulliken |
CHELPG |
AIM |
ESP |
| 1 |
P |
0.222 |
|
|
|
| 2 |
O |
-0.654 |
|
|
|
| 3 |
H |
0.413 |
|
|
|
| 4 |
H |
0.010 |
|
|
|
| 5 |
H |
0.010 |
|
|
|
Electric dipole moments
Electric dipole components in Debye
(What's a Debye? See section
VII.A.3)
| |
x |
y |
z |
Total |
| |
0.712 |
-0.062 |
0.000 |
0.715 |
| CHELPG |
|
|
|
|
| AIM |
|
|
|
|
| ESP |
|
|
|
|
Electric Quadrupole moment
Quadrupole components in D Å
| Primitive |
|---|
| | x | y | z |
|---|
| x |
-19.165 |
3.929 |
0.000 |
| y |
3.929 |
-20.162 |
0.000 |
| z |
0.000 |
0.000 |
-19.931 |
|
| Traceless |
|---|
| | x | y | z |
|---|
| x |
0.882 |
3.929 |
0.000 |
| y |
3.929 |
-0.614 |
0.000 |
| z |
0.000 |
0.000 |
-0.268 |
|
| Polar |
|---|
| 3z2-r2 | -0.536 |
|---|
| x2-y2 | 0.998 |
|---|
| xy | 3.929 |
|---|
| xz | 0.000 |
|---|
| yz | 0.000 |
|---|
|
Polarizabilities
Components of the polarizability tensor.
Units are
Å
3 (Angstrom cubed)
Change units.
| |
x |
y |
z |
| x |
3.528 |
0.692 |
0.000 |
| y |
0.692 |
3.799 |
0.000 |
| z |
0.000 |
0.000 |
3.547 |
<r2> (average value of r
2) Å
2
| <r2> |
35.460 |
| (<r2>)1/2 |
5.955 |