Jump to
S1C2
Energy calculated at B2PLYP=FULLultrafine/cc-pV(T+d)Z
| | hartrees |
|---|
| Energy at 0K | -759.375406 |
| Energy at 298.15K | |
| HF Energy | -758.983377 |
| Nuclear repulsion energy | 182.195239 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at B2PLYP=FULLultrafine/cc-pV(T+d)Z
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A1' |
549 |
549 |
0.00 |
|
|
|
| 2 |
A2" |
424 |
424 |
23.89 |
|
|
|
| 3 |
E' |
573 |
573 |
240.84 |
|
|
|
| 3 |
E' |
573 |
573 |
240.84 |
|
|
|
| 4 |
E' |
85i |
85i |
0.33 |
|
|
|
| 4 |
E' |
85i |
85i |
0.33 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 973.4 cm
-1
Scaled (by 1) Zero Point Vibrational Energy (zpe) 973.4 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
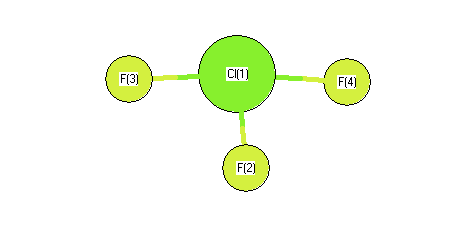
Geometric Data calculated at B2PLYP=FULLultrafine/cc-pV(T+d)Z
Point Group is D3h
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
0.000 |
0.000 |
0.000 |
| F2 |
0.000 |
1.741 |
0.000 |
| F3 |
1.507 |
-0.870 |
0.000 |
| F4 |
-1.507 |
-0.870 |
0.000 |
Atom - Atom Distances (Å)
| |
Cl1 |
F2 |
F3 |
F4 |
| Cl1 | | 1.7406 | 1.7406 | 1.7406 |
F2 | 1.7406 | | 3.0149 | 3.0149 | F3 | 1.7406 | 3.0149 | | 3.0149 | F4 | 1.7406 | 3.0149 | 3.0149 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| F2 |
Cl1 |
F3 |
120.000 |
|
F2 |
Cl1 |
F4 |
120.000 |
| F3 |
Cl1 |
F4 |
120.000 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
Jump to
S1C1
Energy calculated at B2PLYP=FULLultrafine/cc-pV(T+d)Z
| | hartrees |
|---|
| Energy at 0K | -759.403192 |
| Energy at 298.15K | |
| HF Energy | -759.034242 |
| Nuclear repulsion energy | 194.955186 |
The energy at 298.15K was derived from the energy at 0K
and an integrated heat capacity that used the calculated vibrational frequencies.
Vibrational Frequencies calculated at B2PLYP=FULLultrafine/cc-pV(T+d)Z
| Mode Number |
Symmetry |
Frequency
(cm-1) |
Scaled Frequency
(cm-1) |
IR Intensities
(km mol-1) |
Raman Act
(Å4/u) |
Dep P |
Dep U |
|---|
| 1 |
A1 |
761 |
761 |
54.50 |
|
|
|
| 2 |
A1 |
532 |
532 |
3.65 |
|
|
|
| 3 |
A1 |
318 |
318 |
12.56 |
|
|
|
| 4 |
B1 |
333 |
333 |
17.17 |
|
|
|
| 5 |
B2 |
721 |
721 |
473.59 |
|
|
|
| 6 |
B2 |
425 |
425 |
0.19 |
|
|
|
Unscaled Zero Point Vibrational Energy (zpe) 1544.8 cm
-1
Scaled (by 1) Zero Point Vibrational Energy (zpe) 1544.8 cm
-1
See section
III.C.1 List or set vibrational scaling factors
to change the scale factors used here.
See section
III.C.2
Calculate a vibrational scaling factor for a given set of molecules
to determine the least squares best scaling factor.
Geometric Data calculated at B2PLYP=FULLultrafine/cc-pV(T+d)Z
Point Group is C2v
Cartesians (Å)
| Atom |
x (Å) |
y (Å) |
z (Å) |
|---|
| Cl1 |
0.000 |
0.000 |
0.356 |
| F2 |
0.000 |
0.000 |
-1.252 |
| F3 |
0.000 |
1.707 |
0.290 |
| F4 |
0.000 |
-1.707 |
0.290 |
Atom - Atom Distances (Å)
| |
Cl1 |
F2 |
F3 |
F4 |
| Cl1 | | 1.6082 | 1.7083 | 1.7083 |
F2 | 1.6082 | | 2.3003 | 2.3003 | F3 | 1.7083 | 2.3003 | | 3.4140 | F4 | 1.7083 | 2.3003 | 3.4140 | |
 More geometry information
More geometry information
Calculated Bond Angles
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| F2 |
Cl1 |
F3 |
87.776 |
|
F2 |
Cl1 |
F4 |
87.776 |
| F3 |
Cl1 |
F4 |
175.551 |
|
Electronic energy levels
Charges, Dipole, Quadrupole and Polarizability
