Vibrational levels (cm-1) 
| Mode Number |
Symmetry |
Frequency |
Intensity |
Comment |
Description |
| Fundamental(cm-1) |
Harmonic(cm-1) |
Reference |
(km mol-1) |
unc. |
Reference |
| 1 |
A' |
1862 |
|
webbook |
|
|
|
|
CO stretch |
| 2 |
A' |
1026 |
|
|
|
|
|
|
CF str |
| 3 |
A' |
628 |
|
|
|
|
|
|
bend |
vibrational zero-point energy: 1757.6 cm
-1 (from fundamental vibrations)
Calculated vibrational frequencies for
FCO (Carbonyl fluoride).
Geometric Data
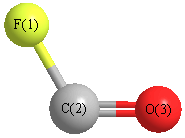
An error occurred on the server when processing the URL. Please contact the system administrator.
If you are the system administrator please click
here to find out more about this error.






 An error occurred on the server when processing the URL. Please contact the system administrator. If you are the system administrator please click
An error occurred on the server when processing the URL. Please contact the system administrator. If you are the system administrator please click