|
Computational Chemistry Comparison and Benchmark DataBase
Release 22 (May 2022) Standard Reference Database 101
National Institute of Standards and Technology
|
|
|
|
You are here: Home > Geometry > Calculated > Calculated geometry OR Calculated > Geometry > Calculated geometry
|
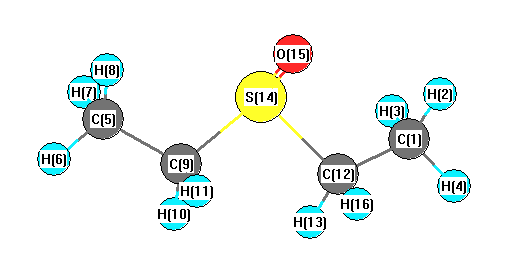
Geometry for C4H10OS (Diethyl sulfoxide)
1A C1
1910171554
InChI=1S/C4H10OS/c1-3-6(5)4-2/h3-4H2,1-2H3 INChIKey=CCAFPWNGIUBUSD-UHFFFAOYSA-N
MP2/CEP-31G*
Point group is C1
| Atom |
Internal |
|
Principal |
| x (Å) |
y (Å) |
z (Å) |
|
a (Å) |
b (Å) |
c (Å) |
| C1 |
1.4266 |
-1.3156 |
-0.5868 |
|
-1.3014 |
-1.4416 |
-0.5818 |
| H2 |
2.1077 |
-2.1674 |
-0.4093 |
|
-1.8996 |
-2.3531 |
-0.4022 |
| H3 |
0.5844 |
-1.6750 |
-1.2017 |
|
-0.4325 |
-1.7207 |
-1.2013 |
| H4 |
1.9737 |
-0.5374 |
-1.1463 |
|
-1.9210 |
-0.7170 |
-1.1374 |
| C5 |
-2.2090 |
-0.5837 |
0.1061 |
|
2.2542 |
-0.3764 |
0.0945 |
| H6 |
-2.5169 |
-0.2981 |
1.1282 |
|
2.5393 |
-0.0646 |
1.1155 |
| H7 |
-3.1191 |
-0.6466 |
-0.5168 |
|
3.1630 |
-0.3539 |
-0.5330 |
| H8 |
-1.7552 |
-1.5883 |
0.1442 |
|
1.8957 |
-1.4188 |
0.1333 |
| C9 |
-1.2417 |
0.4581 |
-0.4971 |
|
1.1914 |
0.5718 |
-0.5024 |
| H10 |
-1.7506 |
1.4240 |
-0.6641 |
|
1.6076 |
1.5809 |
-0.6705 |
| H11 |
-0.8034 |
0.1379 |
-1.4571 |
|
0.7798 |
0.2133 |
-1.4608 |
| C12 |
0.9508 |
-0.7445 |
0.7640 |
|
-0.8738 |
-0.8303 |
0.7675 |
| H13 |
0.2617 |
-1.4178 |
1.3048 |
|
-0.1225 |
-1.4373 |
1.3040 |
| S14 |
0.1495 |
0.9107 |
0.6157 |
|
-0.2303 |
0.8922 |
0.6178 |
| O15 |
1.0998 |
1.7641 |
-0.2403 |
|
-1.2599 |
1.6548 |
-0.2323 |
| H16 |
1.8149 |
-0.5372 |
1.4203 |
|
-1.7501 |
-0.7047 |
1.4283 |
Atom - Atom Distances (Å)
| |
C1 |
H2 |
H3 |
H4 |
C5 |
H6 |
H7 |
H8 |
C9 |
H10 |
H11 |
C12 |
H13 |
S14 |
O15 |
H16 |
| C1 |
|
1.1050 |
1.1030 |
1.1036 |
3.7728 |
4.4190 |
4.5953 |
3.2761 |
3.2053 |
4.1960 |
2.8006 |
1.5418 |
2.2239 |
2.8343 |
3.1163 |
2.1874 |
|---|
| H2 |
1.1050 |
| 1.7863 |
1.7939 |
4.6269 |
5.2197 |
5.4447 |
3.9451 |
4.2567 |
5.2773 |
3.8584 |
2.1771 |
2.6283 |
3.7894 |
4.0622 |
2.4679 |
|---|
| H3 |
1.1030 |
1.7863 |
| 1.7964 |
3.2718 |
4.1161 |
3.9042 |
2.7005 |
2.8950 |
3.9173 |
2.2973 |
2.2054 |
2.5402 |
3.1902 |
3.6080 |
3.1118 |
|---|
| H4 |
1.1036 |
1.7939 |
1.7964 |
| 4.3664 |
5.0394 |
5.1327 |
4.0834 |
3.4280 |
4.2367 |
2.8749 |
2.1768 |
3.1167 |
2.9205 |
2.6233 |
2.5715 |
|---|
| C5 |
3.7728 |
4.6269 |
3.2718 |
4.3664 |
|
1.1050 |
1.1047 |
1.1030 |
1.5443 |
2.1987 |
2.2227 |
3.2316 |
2.8700 |
2.8383 |
4.0720 |
4.2333 |
|---|
| H6 |
4.4190 |
5.2197 |
4.1161 |
5.0394 |
1.1050 |
| 1.7861 |
1.7925 |
2.1999 |
2.6010 |
3.1321 |
3.5152 |
3.0009 |
2.9722 |
4.3825 |
4.3482 |
|---|
| H7 |
4.5953 |
5.4447 |
3.9042 |
5.1327 |
1.1047 |
1.7861 |
| 1.7844 |
2.1784 |
2.4864 |
2.6196 |
4.2678 |
3.9170 |
3.7937 |
4.8670 |
5.3018 |
|---|
| H8 |
3.2761 |
3.9451 |
2.7005 |
4.0834 |
1.1030 |
1.7925 |
1.7844 |
| 2.2051 |
3.1189 |
2.5397 |
2.9014 |
2.3332 |
3.1773 |
4.4202 |
3.9343 |
|---|
| C9 |
3.2053 |
4.2567 |
2.8950 |
3.4280 |
1.5443 |
2.1999 |
2.1784 |
2.2051 |
|
1.1045 |
1.1029 |
2.8007 |
3.0043 |
1.8381 |
2.6935 |
3.7429 |
|---|
| H10 |
4.1960 |
5.2773 |
3.9173 |
4.2367 |
2.1987 |
2.6010 |
2.4864 |
3.1189 |
1.1045 |
| 1.7833 |
3.7469 |
4.0002 |
2.3477 |
2.9018 |
4.5720 |
|---|
| H11 |
2.8006 |
3.8584 |
2.2973 |
2.8749 |
2.2227 |
3.1321 |
2.6196 |
2.5397 |
1.1029 |
1.7833 |
| 2.9647 |
3.3441 |
2.4087 |
2.7835 |
3.9485 |
|---|
| C12 |
1.5418 |
2.1771 |
2.2054 |
2.1768 |
3.2316 |
3.5152 |
4.2678 |
2.9014 |
2.8007 |
3.7469 |
2.9647 |
|
1.1048 |
1.8449 |
2.7063 |
1.1047 |
|---|
| H13 |
2.2239 |
2.6283 |
2.5402 |
3.1167 |
2.8700 |
3.0009 |
3.9170 |
2.3332 |
3.0043 |
4.0002 |
3.3441 |
1.1048 |
| 2.4309 |
3.6352 |
1.7892 |
|---|
| S14 |
2.8343 |
3.7894 |
3.1902 |
2.9205 |
2.8383 |
2.9722 |
3.7937 |
3.1773 |
1.8381 |
2.3477 |
2.4087 |
1.8449 |
2.4309 |
| 1.5376 |
2.3488 |
|---|
| O15 |
3.1163 |
4.0622 |
3.6080 |
2.6233 |
4.0720 |
4.3825 |
4.8670 |
4.4202 |
2.6935 |
2.9018 |
2.7835 |
2.7063 |
3.6352 |
1.5376 |
| 2.9265 |
|---|
| H16 |
2.1874 |
2.4679 |
3.1118 |
2.5715 |
4.2333 |
4.3482 |
5.3018 |
3.9343 |
3.7429 |
4.5720 |
3.9485 |
1.1047 |
1.7892 |
2.3488 |
2.9265 |
|
|---|
Maximum atom distance is 5.4447Å
between atoms H2 and H7.
Calculated Bond Angles (degrees) (Ignoring Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
C12 |
S14 |
113.321 |
|
C5 |
C9 |
S14 |
113.812 |
|
C9 |
S14 |
C12 |
99.002 |
|
C9 |
S14 |
O15 |
105.512 |
|
C12 |
S14 |
O15 |
105.922 |
|
Calculated Bond Angles (degrees) (Involving Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
C12 |
H13 |
113.309 |
|
C1 |
C12 |
H16 |
110.398 |
|
H2 |
C1 |
H3 |
107.999 |
|
H2 |
C1 |
H4 |
108.633 |
|
H2 |
C1 |
C12 |
109.572 |
|
H3 |
C1 |
H4 |
109.005 |
|
H3 |
C1 |
C12 |
111.926 |
|
H4 |
C1 |
C12 |
109.636 |
|
C5 |
C9 |
H10 |
111.129 |
|
C5 |
C9 |
H11 |
113.149 |
|
H6 |
C5 |
H7 |
107.864 |
|
H6 |
C5 |
H8 |
108.548 |
|
H6 |
C5 |
C9 |
111.190 |
|
H7 |
C5 |
H8 |
107.857 |
|
H7 |
C5 |
C9 |
109.522 |
|
H8 |
C5 |
C9 |
111.727 |
|
H10 |
C9 |
H11 |
107.782 |
|
H10 |
C9 |
S14 |
102.998 |
|
H11 |
C9 |
S14 |
107.316 |
|
H13 |
C12 |
S14 |
108.373 |
|
H13 |
C12 |
H16 |
108.150 |
|
S14 |
C12 |
H16 |
102.653 |
For information on specific bond angles or dihedrals
see the geometry comparison page in section
Comparisons > Geometry > Bonds, angles.