|
Computational Chemistry Comparison and Benchmark DataBase
Release 22 (May 2022) Standard Reference Database 101
National Institute of Standards and Technology
|
|
|
|
You are here: Home > Geometry > Calculated > Calculated geometry OR Calculated > Geometry > Calculated geometry
|
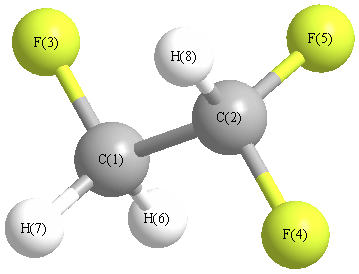
Geometry for CHF2CH2F (Ethane, 1,1,2-trifluoro)
1A C1
1910171554
InChI=1S/C2H3F3/c3-1-2(4)5/h2H,1H2 INChIKey=WGZYQOSEVSXDNI-UHFFFAOYSA-N
HF/LANL2DZ
Point group is C1
| Atom |
Internal |
|
Principal |
| x (Å) |
y (Å) |
z (Å) |
|
a (Å) |
b (Å) |
c (Å) |
| C1 |
-0.7803 |
-0.5448 |
-0.3573 |
|
0.8011 |
-0.5137 |
-0.3573 |
| C2 |
0.4445 |
0.0158 |
0.3268 |
|
-0.4498 |
-0.0047 |
0.3198 |
| F3 |
-1.9149 |
0.1008 |
0.1961 |
|
1.9080 |
0.1500 |
0.2297 |
| F4 |
1.5254 |
-0.8144 |
0.0253 |
|
-1.5017 |
-0.8575 |
-0.0189 |
| F5 |
0.7547 |
1.2716 |
-0.1810 |
|
-0.7879 |
1.2537 |
-0.1631 |
| H6 |
-0.7610 |
-0.3450 |
-1.4148 |
|
0.7926 |
-0.2891 |
-1.4099 |
| H7 |
-0.8697 |
-1.5992 |
-0.1606 |
|
0.9175 |
-1.5697 |
-0.1842 |
| H8 |
0.3587 |
0.0968 |
1.3942 |
|
-0.3831 |
0.0530 |
1.3900 |
Atom - Atom Distances (Å)
| |
C1 |
C2 |
F3 |
F4 |
F5 |
H6 |
H7 |
H8 |
| C1 |
|
1.5107 |
1.4179 |
2.3528 |
2.3846 |
1.0763 |
1.0764 |
2.1856 |
|---|
| C2 |
1.5107 |
| 2.3646 |
1.3959 |
1.3896 |
2.1486 |
2.1384 |
1.0739 |
|---|
| F3 |
1.4179 |
2.3646 |
| 3.5641 |
2.9394 |
2.0310 |
2.0273 |
2.5700 |
|---|
| F4 |
2.3528 |
1.3959 |
3.5641 |
| 2.2334 |
2.7426 |
2.5273 |
2.0163 |
|---|
| F5 |
2.3846 |
1.3896 |
2.9394 |
2.2334 |
| 2.5363 |
3.2986 |
2.0045 |
|---|
| H6 |
1.0763 |
2.1486 |
2.0310 |
2.7426 |
2.5363 |
| 1.7770 |
3.0560 |
|---|
| H7 |
1.0764 |
2.1384 |
2.0273 |
2.5273 |
3.2986 |
1.7770 |
| 2.6083 |
|---|
| H8 |
2.1856 |
1.0739 |
2.5700 |
2.0163 |
2.0045 |
3.0560 |
2.6083 |
|
|---|
Maximum atom distance is 3.5641Å
between atoms F3 and F4.
Calculated Bond Angles (degrees) (Ignoring Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
C2 |
F4 |
108.017 |
|
C1 |
C2 |
F5 |
110.538 |
|
C2 |
C1 |
F3 |
107.644 |
|
F4 |
C2 |
F5 |
106.600 |
Calculated Bond Angles (degrees) (Involving Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
C2 |
H8 |
114.415 |
|
C2 |
C1 |
H6 |
111.191 |
|
C2 |
C1 |
H7 |
110.370 |
|
F3 |
C1 |
H6 |
108.259 |
|
F3 |
C1 |
H7 |
107.953 |
|
F4 |
C2 |
H8 |
108.747 |
|
F5 |
C2 |
H8 |
108.230 |
|
H6 |
C1 |
H7 |
111.274 |
For information on specific bond angles or dihedrals
see the geometry comparison page in section
Comparisons > Geometry > Bonds, angles.