|
Computational Chemistry Comparison and Benchmark DataBase
Release 22 (May 2022) Standard Reference Database 101
National Institute of Standards and Technology
|
|
|
|
You are here: Home > Geometry > Calculated > Calculated geometry OR Calculated > Geometry > Calculated geometry
|
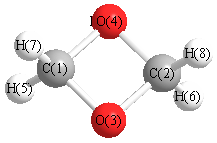
Geometry for C2H4O2 (1,3-dioxetane)
1Ag D2H
1910171554
InChI=1S/C2H4O2/c1-3-2-4-1/h1-2H2 INChIKey=GFAJOMHUNNCCJQ-UHFFFAOYSA-N
G3B3
This model chemistry uses a geometry from
B3LYP/6-31G*
Point group is D2h
| Atom |
Internal |
|
Principal |
| x (Å) |
y (Å) |
z (Å) |
|
a (Å) |
b (Å) |
c (Å) |
| C1 |
-0.9815 |
0.0000 |
0.0000 |
|
0.0000 |
-0.9815 |
0.0000 |
| C2 |
0.9815 |
0.0000 |
0.0000 |
|
0.0000 |
0.9815 |
0.0000 |
| O3 |
0.0000 |
1.0379 |
0.0000 |
|
1.0379 |
0.0000 |
0.0000 |
| O4 |
0.0000 |
-1.0379 |
0.0000 |
|
-1.0379 |
0.0000 |
0.0000 |
| H5 |
-1.6082 |
0.0000 |
0.9044 |
|
0.0000 |
-1.6082 |
0.9044 |
| H6 |
1.6082 |
0.0000 |
0.9044 |
|
0.0000 |
1.6082 |
0.9044 |
| H7 |
-1.6082 |
0.0000 |
-0.9044 |
|
0.0000 |
-1.6082 |
-0.9044 |
| H8 |
1.6082 |
0.0000 |
-0.9044 |
|
0.0000 |
1.6082 |
-0.9044 |
Atom - Atom Distances (Å)
| |
C1 |
C2 |
O3 |
O4 |
H5 |
H6 |
H7 |
H8 |
| C1 |
| 1.9629 |
1.4285 |
1.4285 |
1.1003 |
2.7431 |
1.1003 |
2.7431 |
|---|
| C2 |
1.9629 |
|
1.4285 |
1.4285 |
2.7431 |
1.1003 |
2.7431 |
1.1003 |
|---|
| O3 |
1.4285 |
1.4285 |
| 2.0759 |
2.1170 |
2.1170 |
2.1170 |
2.1170 |
|---|
| O4 |
1.4285 |
1.4285 |
2.0759 |
| 2.1170 |
2.1170 |
2.1170 |
2.1170 |
|---|
| H5 |
1.1003 |
2.7431 |
2.1170 |
2.1170 |
| 3.2165 |
1.8087 |
3.6901 |
|---|
| H6 |
2.7431 |
1.1003 |
2.1170 |
2.1170 |
3.2165 |
| 3.6901 |
1.8087 |
|---|
| H7 |
1.1003 |
2.7431 |
2.1170 |
2.1170 |
1.8087 |
3.6901 |
| 3.2165 |
|---|
| H8 |
2.7431 |
1.1003 |
2.1170 |
2.1170 |
3.6901 |
1.8087 |
3.2165 |
|
|---|
Maximum atom distance is 3.6901Å
between atoms H5 and H8.
Calculated Bond Angles (degrees) (Ignoring Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
O3 |
C2 |
86.802 |
|
C1 |
O4 |
C2 |
86.802 |
|
O3 |
C1 |
O4 |
93.198 |
|
O3 |
C2 |
O4 |
93.198 |
Calculated Bond Angles (degrees) (Involving Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
O3 |
C1 |
H5 |
113.053 |
|
O3 |
C1 |
H7 |
113.053 |
|
O3 |
C2 |
H6 |
113.053 |
|
O3 |
C2 |
H8 |
113.053 |
|
O4 |
C1 |
H5 |
113.053 |
|
O4 |
C1 |
H7 |
113.053 |
|
O4 |
C2 |
H6 |
113.053 |
|
O4 |
C2 |
H8 |
113.053 |
|
H5 |
C1 |
H7 |
110.514 |
|
H6 |
C2 |
H8 |
110.514 |
For information on specific bond angles or dihedrals
see the geometry comparison page in section
Comparisons > Geometry > Bonds, angles.