|
Computational Chemistry Comparison and Benchmark DataBase
Release 22 (May 2022) Standard Reference Database 101
National Institute of Standards and Technology
|
|
|
|
You are here: Home > Geometry > Calculated > Calculated geometry OR Calculated > Geometry > Calculated geometry
|
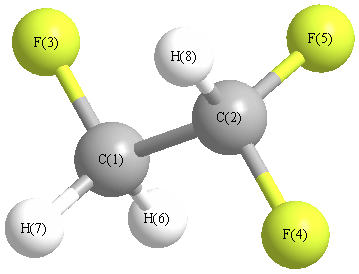
Geometry for CHF2CH2F (Ethane, 1,1,2-trifluoro)
1A C1
1910171554
InChI=1S/C2H3F3/c3-1-2(4)5/h2H,1H2 INChIKey=WGZYQOSEVSXDNI-UHFFFAOYSA-N
BLYP/aug-cc-pVDZ
Point group is C1
| Atom |
Internal |
|
Principal |
| x (Å) |
y (Å) |
z (Å) |
|
a (Å) |
b (Å) |
c (Å) |
| C1 |
-0.7809 |
-0.5918 |
-0.3053 |
|
-0.7594 |
-0.6046 |
-0.3332 |
| C2 |
0.4648 |
0.0194 |
0.3337 |
|
0.4588 |
0.0233 |
0.3417 |
| F3 |
-1.9208 |
0.1058 |
0.1630 |
|
-1.9254 |
0.0493 |
0.1342 |
| F4 |
1.5594 |
-0.7857 |
-0.0006 |
|
1.5803 |
-0.7427 |
0.0049 |
| F5 |
0.7052 |
1.2879 |
-0.1843 |
|
0.6722 |
1.3110 |
-0.1395 |
| H6 |
-0.7361 |
-0.4939 |
-1.4050 |
|
-0.7001 |
-0.4769 |
-1.4292 |
| H7 |
-0.8765 |
-1.6509 |
-0.0040 |
|
-0.8305 |
-1.6734 |
-0.0608 |
| H8 |
0.4161 |
0.1067 |
1.4348 |
|
0.3904 |
0.0804 |
1.4437 |
Atom - Atom Distances (Å)
| |
C1 |
C2 |
F3 |
F4 |
F5 |
H6 |
H7 |
H8 |
| C1 |
|
1.5276 |
1.4162 |
2.3679 |
2.3993 |
1.1050 |
1.1052 |
2.2245 |
|---|
| C2 |
1.5276 |
| 2.3933 |
1.3993 |
1.3912 |
2.1746 |
2.1686 |
1.1056 |
|---|
| F3 |
1.4162 |
2.3933 |
| 3.5963 |
2.9007 |
2.0548 |
2.0505 |
2.6606 |
|---|
| F4 |
2.3679 |
1.3993 |
3.5963 |
| 2.2501 |
2.7068 |
2.5850 |
2.0405 |
|---|
| F5 |
2.3993 |
1.3912 |
2.9007 |
2.2501 |
| 2.5966 |
3.3423 |
2.0249 |
|---|
| H6 |
1.1050 |
2.1746 |
2.0548 |
2.7068 |
2.5966 |
| 1.8224 |
3.1230 |
|---|
| H7 |
1.1052 |
2.1686 |
2.0505 |
2.5850 |
3.3423 |
1.8224 |
| 2.6134 |
|---|
| H8 |
2.2245 |
1.1056 |
2.6606 |
2.0405 |
2.0249 |
3.1230 |
2.6134 |
|
|---|
Maximum atom distance is 3.5963Å
between atoms F3 and F4.
Calculated Bond Angles (degrees) (Ignoring Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
C2 |
F4 |
107.924 |
|
C1 |
C2 |
F5 |
110.486 |
|
C2 |
C1 |
F3 |
108.718 |
|
F4 |
C2 |
F5 |
107.486 |
Calculated Bond Angles (degrees) (Involving Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
C2 |
H8 |
114.352 |
|
C2 |
C1 |
H6 |
110.357 |
|
C2 |
C1 |
H7 |
109.870 |
|
F3 |
C1 |
H6 |
108.549 |
|
F3 |
C1 |
H7 |
108.194 |
|
F4 |
C2 |
H8 |
108.529 |
|
F5 |
C2 |
H8 |
107.843 |
|
H6 |
C1 |
H7 |
111.084 |
For information on specific bond angles or dihedrals
see the geometry comparison page in section
Comparisons > Geometry > Bonds, angles.