|
Computational Chemistry Comparison and Benchmark DataBase
Release 22 (May 2022) Standard Reference Database 101
National Institute of Standards and Technology
|
|
|
|
You are here: Home > Geometry > Calculated > Calculated geometry OR Calculated > Geometry > Calculated geometry
|
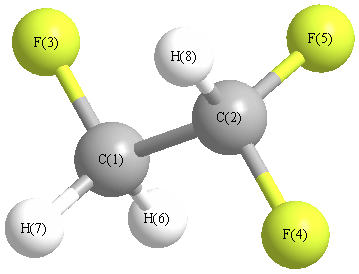
Geometry for CHF2CH2F (Ethane, 1,1,2-trifluoro)
1A C1
1910171554
InChI=1S/C2H3F3/c3-1-2(4)5/h2H,1H2 INChIKey=WGZYQOSEVSXDNI-UHFFFAOYSA-N
PBE1PBE/6-311G**
Point group is C1
| Atom |
Internal |
|
Principal |
| x (Å) |
y (Å) |
z (Å) |
|
a (Å) |
b (Å) |
c (Å) |
| C1 |
-0.7726 |
-0.5844 |
-0.2844 |
|
-0.7515 |
-0.5979 |
-0.3116 |
| C2 |
0.4658 |
0.0206 |
0.3263 |
|
0.4599 |
0.0249 |
0.3342 |
| F3 |
-1.8767 |
0.1085 |
0.1517 |
|
-1.8811 |
0.0527 |
0.1242 |
| F4 |
1.5259 |
-0.7619 |
-0.0064 |
|
1.5466 |
-0.7191 |
-0.0013 |
| F5 |
0.6833 |
1.2565 |
-0.1806 |
|
0.6505 |
1.2792 |
-0.1375 |
| H6 |
-0.7054 |
-0.5168 |
-1.3738 |
|
-0.6695 |
-0.5003 |
-1.3977 |
| H7 |
-0.8568 |
-1.6296 |
0.0251 |
|
-0.8112 |
-1.6527 |
-0.0302 |
| H8 |
0.4113 |
0.1016 |
1.4159 |
|
0.3865 |
0.0761 |
1.4245 |
Atom - Atom Distances (Å)
| |
C1 |
C2 |
F3 |
F4 |
F5 |
H6 |
H7 |
H8 |
| C1 |
|
1.5075 |
1.3745 |
2.3221 |
2.3493 |
1.0936 |
1.0932 |
2.1825 |
|---|
| C2 |
1.5075 |
| 2.3506 |
1.3590 |
1.3535 |
2.1333 |
2.1361 |
1.0940 |
|---|
| F3 |
1.3745 |
2.3506 |
| 3.5157 |
2.8252 |
2.0224 |
2.0192 |
2.6141 |
|---|
| F4 |
2.3221 |
1.3590 |
3.5157 |
| 2.1942 |
2.6284 |
2.5360 |
2.0027 |
|---|
| F5 |
2.3493 |
1.3535 |
2.8252 |
2.1942 |
| 2.5489 |
3.2778 |
1.9892 |
|---|
| H6 |
1.0936 |
2.1333 |
2.0224 |
2.6284 |
2.5489 |
| 1.7939 |
3.0679 |
|---|
| H7 |
1.0932 |
2.1361 |
2.0192 |
2.5360 |
3.2778 |
1.7939 |
| 2.5572 |
|---|
| H8 |
2.1825 |
1.0940 |
2.6141 |
2.0027 |
1.9892 |
3.0679 |
2.5572 |
|
|---|
Maximum atom distance is 3.5157Å
between atoms F3 and F4.
Calculated Bond Angles (degrees) (Ignoring Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
C2 |
F4 |
108.094 |
|
C1 |
C2 |
F5 |
110.288 |
|
C2 |
C1 |
F3 |
109.209 |
|
F4 |
C2 |
F5 |
107.984 |
Calculated Bond Angles (degrees) (Involving Hydrogens)
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
|
C1 |
C2 |
H8 |
113.100 |
|
C2 |
C1 |
H6 |
109.165 |
|
C2 |
C1 |
H7 |
109.406 |
|
F3 |
C1 |
H6 |
109.527 |
|
F3 |
C1 |
H7 |
109.282 |
|
F4 |
C2 |
H8 |
108.984 |
|
F5 |
C2 |
H8 |
108.266 |
|
H6 |
C1 |
H7 |
110.235 |
For information on specific bond angles or dihedrals
see the geometry comparison page in section
Comparisons > Geometry > Bonds, angles.