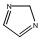
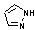Methods with standard basis sets
|
|
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
cc-pV(T+d)Z |
daug-cc-pVTZ |
| hartree fock |
HF |
0.0 a
-28.1 b
3.5 c
279.9 d |
0.0 a
-61.3 b
29.2 c
231.8 d |
0.0 a
-61.3 b
29.2 c
231.8 d |
0.0 a
-50.8 b
42.7 c
229.9 d |
0.0 a
-55.2 b
-14.0 c
179.9 d |
0.0 a
-55.4 b
-6.6 c
187.1 d |
0.0 a
-56.0 b
-2.5 c
188.4 d |
0.0 a
-54.7 b
-11.6 c
181.1 d |
0.0 a
-55.4 b
-4.7 c
187.8 d |
0.0 a
-55.7 b
-9.6 c
182.9 d |
0.0 a
-57.8 b
180.0 d |
0.0 a
-55.4 b
-2.2 c
186.0 d |
0.0 a
-54.3 b
-5.8 c
192.6 d |
0.0 a
-55.8 b
-4.0 c
183.9 d |
0.0 a
-55.3 b
-8.6 c
187.9 d |
0.0 a
-56.3 b
-4.0 c
182.3 d |
0.0 a
-55.8 b |
0.0 a
-56.3 b
-3.9 c
3.5 d |
| density functional |
LSDA |
NC
NC |
NC
NC
NC |
NC
NC |
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
| BLYP |
0.0 a
-14.5 b
22.8 c
368.4 d |
0.0 a
-42.1 b
35.0 c
276.5 d |
0.0 a
-42.1 b
35.0 c
276.5 d |
0.0 a
-35.5 b
45.5 c
270.6 d |
0.0 a
-38.7 b
14.0 c
11.1 d |
0.0 a
-38.9 b
20.3 c
235.0 d |
0.0 a
-39.8 b
23.0 c
230.0 d |
0.0 a
-40.0 b
19.9 c
228.4 d |
0.0 a
-40.5 b
26.6 c
234.9 d |
0.0 a
-39.8 b
23.7 c
231.2 d |
|
NC
NC
NC |
0.0 a
-38.5 b
26.9 c
243.4 d |
0.0 a
-40.8 b
27.6 c
227.5 d |
0.0 a
-39.5 b
25.8 c
232.5 d |
NC
NC
NC |
|
|
| B1B95 |
0.0 a
-15.5 b
37.9 c
335.7 d |
0.0 a
-38.0 b
56.1 c
266.2 d |
0.0 a
-38.0 b
56.1 c
266.2 d |
0.0 a
-27.5 b
71.2 c
265.7 d |
0.0 a
-43.1 b
20.5 c
210.2 d |
0.0 a
-12.0 b
36.8 c
241.3 d |
0.0 a
-33.6 b
39.6 c
224.5 d |
0.0 a
-33.6 b
34.0 c
219.7 d |
0.0 a
-34.2 b
40.9 c
226.2 d |
0.0 a
-34.6 b
38.2 c
223.6 d |
|
NC
NC
NC |
0.0 a
-33.4 b
39.7 c
232.0 d |
0.0 a
-44.3 b
32.2 c
211.1 d |
0.0 a
-43.5 b
28.9 c
215.2 d |
NC
NC
NC |
0.0 a
-44.3 b |
|
| B3LYP |
0.0 a
-18.4 b
26.2 c
344.3 d |
0.0 a
-46.9 b
39.5 c
261.9 d |
0.0 a
-46.9 b
39.5 c
261.9 d |
0.0 a
-39.1 b
50.8 c
257.9 d |
0.0 a
-42.3 b
14.4 c
215.5 d |
0.0 a
-42.5 b
21.0 c
222.1 d |
0.0 a
-43.3 b
23.9 c
219.0 d |
0.0 a
-43.3 b
19.5 c
215.4 d |
0.0 a
-43.8 b
26.4 c
222.1 d |
0.0 a
-43.2 b
23.6 c
218.8 d |
0.0 a
-45.5 b
211.1 d |
0.0 a
-43.9 b
27.0 c
217.1 d |
0.0 a
-41.8 b
26.4 c
230.0 d |
0.0 a
-44.1 b
27.6 c
215.9 d |
0.0 a
-42.9 b
25.3 c
221.2 d |
0.0 a
-44.4 b
27.5 c
212.7 d |
0.0 a
-44.1 b |
|
| B3LYPultrafine |
|
NC
NC
NC |
|
|
0.0 a
-42.2 b
14.5 c
215.5 d |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
|
NC
NC
NC |
NC
NC
NC |
0.0 a
-44.1 b
27.6 c
216.0 d |
NC
NC
NC |
0.0 a
-44.4 b
27.5 c
212.8 d |
|
|
| B3PW91 |
0.0 a
-17.9 b
31.0 c
342.7 d |
0.0 a
-46.8 b
45.2 c
264.2 d |
0.0 a
-46.8 b
45.2 c
264.2 d |
0.0 a
-38.6 b
57.1 c
260.0 d |
0.0 a
-41.2 b
21.0 c
217.7 d |
0.0 a
-41.5 b
27.6 c
224.3 d |
0.0 a
-42.0 b
29.6 c
221.2 d |
0.0 a
-42.4 b
24.3 c
216.2 d |
0.0 a
-42.9 b
31.3 c
222.9 d |
0.0 a
-41.9 b
29.4 c
221.7 d |
|
NC
NC
NC |
0.0 a
-40.9 b
30.9 c
230.2 d |
0.0 a
-42.6 b
32.8 c
218.1 d |
0.0 a
-41.6 b
29.4 c
222.2 d |
NC
NC
NC |
0.0 a
-42.6 b |
|
| mPW1PW91 |
0.0 a
-18.6 b
31.4 c
337.6 d |
0.0 a
-52.4 b
41.5 c
257.2 d |
0.0 a
-48.0 b
45.9 c
261.6 d |
0.0 a
-39.3 b
58.1 c
258.0 d |
0.0 a
-46.1 b
16.7 c
211.2 d |
0.0 a
-46.3 b
23.5 c
218.0 d |
0.0 a
-46.8 b
25.8 c
215.3 d |
0.0 a
-47.1 b
19.8 c
210.0 d |
0.0 a
-43.6 b
31.0 c
220.9 d |
0.0 a
-42.5 b
29.0 c
219.6 d |
|
NC
NC
NC |
0.0 a
-45.8 b
26.2 c
223.5 d |
0.0 a
-43.1 b
32.6 c
216.5 d |
0.0 a
-42.3 b
28.8 c
220.0 d |
NC
NC
NC |
0.0 a
-43.1 b |
|
| M06-2X |
NC
NC
NC |
NC
NC
NC |
0.0 a
-53.7 b
39.9 c
41.9 d |
NC
NC
NC |
0.0 a
-47.3 b
17.8 c
213.4 d |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
| PBEPBE |
0.0 a
-14.4 b
32.1 c
364.1 d |
0.0 a
-42.7 b
42.7 c
277.9 d |
0.0 a
-42.7 b
42.7 c
277.9 d |
0.0 a
-35.1 b
54.3 c
272.5 d |
0.0 a
-37.3 b
23.3 c
230.7 d |
0.0 a
-37.6 b
29.8 c
237.3 d |
0.0 a
-38.2 b
31.9 c
232.3 d |
0.0 a
-38.8 b
27.3 c
229.1 d |
0.0 a
-39.3 b
34.3 c
235.9 d |
0.0 a
-38.1 b
32.2 c
234.3 d |
NC
NC |
NC
NC
NC |
0.0 a
-37.3 b
34.3 c
243.8 d |
0.0 a
-39.0 b
35.9 c
230.2 d |
0.0 a
-37.8 b
33.0 c
233.8 d |
NC
NC
NC |
0.0 a
-39.0 b |
|
| PBEPBEultrafine |
|
NC
NC
NC |
|
|
0.0 a
-37.3 b
23.3 c
230.7 d |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
| PBE1PBE |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
0.0 a
-41.3 b
22.2 c
217.2 d |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
| HSEh1PBE |
NC
NC
NC |
0.0 a
-47.9 b
46.7 c
262.2 d |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
0.0 a
-42.1 b
31.0 c
220.4 d |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
0.0 a
-42.6 b
34.0 c
217.8 d |
NC
NC
NC |
NC
NC
NC |
|
|
| TPSSh |
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
0.0 a
-39.8 b
16.6 c
218.5 d |
NC
NC
NC |
0.0 a
-40.8 b
25.1 c
221.0 d |
NC
NC
NC |
NC
NC |
0.0 a
-40.5 b
24.4 c
23.7 d |
|
NC
NC
NC |
NC
NC
NC |
0.0 a
-41.3 b
28.0 c
218.4 d |
NC
NC
NC |
NC
NC
NC |
|
|
| wB97X-D |
NC
NC
NC |
NC
NC
NC |
0.0 a
-48.7 b
47.1 c
45.2 d |
NC
NC
NC |
0.0 a
-43.4 b
17.7 c
18.3 d |
NC
NC
NC |
0.0 a
-44.2 b
26.1 c
28.0 d |
NC
NC
NC |
0.0 a
-44.9 b
27.8 c
29.1 d |
NC
NC
NC |
|
0.0 a
-44.6 b
28.5 c
30.0 d |
0.0 a
-48.3 b
26.1 c
28.0 d |
0.0 a
-44.8 b
28.6 c
31.2 d |
NC
NC
NC |
0.0 a
-45.2 b
28.0 c
30.9 d |
|
|
| B97D3 |
|
0.0 a
-42.5 b
38.3 c
33.8 d |
|
|
0.0 a
-38.2 b
17.4 c
14.5 d |
|
0.0 a
-39.2 b
26.1 c
29.9 d |
|
0.0 a
-39.7 b
28.4 c
26.3 d |
|
0.0 a
-41.3 b
28.2 c
219.7 d |
0.0 a
-39.7 b
28.9 c
226.4 d |
|
0.0 a
-39.8 b
29.9 c
29.0 d |
|
0.0 a
-40.2 b
29.5 c
29.0 d |
|
|
| |
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
cc-pV(T+d)Z |
daug-cc-pVTZ |
|---|
| Moller Plesset perturbation |
MP2 |
0.0 a
-19.0 b
-8.3 c
338.1 d |
0.0 a
-43.1 b
30.4 c
281.7 d |
0.0 a
-43.1 b
30.4 c
281.7 d |
0.0 a
-35.8 b
42.0 c
275.6 d |
0.0 a
-41.4 b
22.3 c
18.7 d |
0.0 a
-41.7 b
27.5 c
234.6 d |
NC
NC
NC |
0.0 a
-41.3 b
26.1 c
22.3 d |
0.0 a
-42.2 b
30.6 c
236.1 d |
0.0 a
-42.1 b
33.6 c
233.1 d |
|
0.0 a
-42.9 b
30.5 c
229.4 d |
0.0 a
-40.8 b
30.1 c
246.9 d |
0.0 a
-42.4 b
37.2 c
232.4 d |
NC
NC
NC |
NC
NC |
0.0 a
-42.4 b |
|
| MP2=FULL |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
0.0 a
-41.8 b
23.0 c
227.5 d |
0.0 a
-42.1 b
28.2 c
233.1 d |
0.0 a
-42.8 b
31.1 c
230.4 d |
0.0 a
-41.4 b
27.3 c
229.8 d |
0.0 a
-42.4 b
31.7 c
235.0 d |
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
| MP3 |
|
|
|
|
0.0 a
-45.5 b
6.5 c
212.8 d |
|
NC
NC
NC |
|
|
|
|
NC
NC
NC |
NC
NC
NC |
NC
NC |
|
|
|
|
| MP3=FULL |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
0.0 a
-45.9 b
7.1 c
211.4 d |
NC
NC
NC |
0.0 a
-47.0 b
15.2 c
215.5 d |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
|
| MP4 |
|
NC
NC
NC |
|
|
NC
NC
NC |
|
|
|
NC
NC
NC |
|
|
NC
NC
NC |
NC
NC |
NC
NC
NC |
NC
NC |
NC
NC |
|
|
| MP4=FULL |
|
NC
NC
NC |
|
|
NC
NC |
|
|
|
NC
NC |
|
|
|
NC
NC
NC |
NC
NC |
NC
NC |
|
|
|
| B2PLYP |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
0.0 a
-44.2 b
27.1 c
219.0 d |
NC
NC
NC |
NC
NC
NC |
|
|
| B2PLYP=FULL |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC |
|
|
| B2PLYP=FULLultrafine |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
|
|
NC
NC
NC |
|
|
|
| Configuration interaction |
CID |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
0.0 a
-49.2 b
3.1 c
200.3 d |
|
|
NC
NC
NC |
|
|
|
|
|
|
|
|
|
|
| CISD |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
0.0 a
-49.2 b
1.9 c
199.8 d |
|
|
NC
NC
NC |
|
|
|
|
|
|
|
|
|
|
| |
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
cc-pV(T+d)Z |
daug-cc-pVTZ |
|---|
| Quadratic configuration interaction |
QCISD |
|
0.0 a
-47.5 b
16.2 c
259.9 d |
NC
NC
NC |
NC
NC
NC |
0.0 a
-46.0 b
-1.8 c
206.7 d |
0.0 a
-46.4 b
3.4 c
212.3 d |
0.0 a
-47.3 b
6.2 c
209.9 d |
NC
NC
NC |
0.0 a
-46.4 b
5.5 c
213.2 d |
NC
NC
NC |
|
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
|
| QCISD(T) |
|
|
|
|
NC
NC
NC |
|
|
NC
NC
NC |
|
|
|
NC
NC
NC |
NC
NC |
NC
NC
NC |
|
|
|
|
| QCISD(T)=FULL |
|
|
|
|
NC
NC
NC |
|
NC
NC
NC |
|
|
|
|
|
NC
NC |
NC
NC |
|
|
|
|
| Coupled Cluster |
CCD |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
0.0 a
-45.9 b
2.9 c
207.5 d |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
|
| CCSD |
|
|
|
|
NC
NC
NC |
|
|
|
|
NC
NC
NC |
|
NC
NC
NC |
NC
NC
NC |
NC
NC |
NC
NC
NC |
|
|
|
| CCSD=FULL |
|
|
|
|
NC
NC
NC |
|
|
|
|
NC
NC
NC |
|
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
|
| CCSD(T) |
|
|
|
|
NC
NC
NC |
NC
NC
NC |
|
NC
NC
NC |
|
|
|
NC
NC
NC |
NC
NC
NC |
NC
NC |
NC
NC
NC |
|
|
|
| CCSD(T)=FULL |
|
|
|
|
NC
NC
NC |
|
|
|
|
|
|
NC
NC
NC |
NC
NC
NC |
NC
NC |
NC
NC |
|
|
|
| |
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
cc-pV(T+d)Z |
daug-cc-pVTZ |
|---|