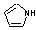
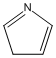
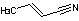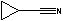Methods with standard basis sets
|
|
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
cc-pV(T+d)Z |
daug-cc-pVTZ |
| hartree fock |
HF |
0.0 b
24.0 c
25.3 d
29.0 e |
0.0 b
-5.3 c
-4.9 d
59.5 e |
0.0 b
-5.3 c
-4.9 d
59.5 e |
0.0 b
13.7 c
14.6 d
79.0 e |
0.0 b
-15.3 c
-14.1 d
28.8 e
41.6 f
34.9 g |
0.0 b
-7.0 c
-5.9 d
36.9 e |
0.0 b
2.0 c
3.1 d
47.5 e |
0.0 b
-17.9 c
-16.6 d
30.6 e |
0.0 b
-9.1 c
-8.0 d
38.6 e |
0.0 b
-10.4 c
-9.2 d
35.1 e |
0.0 b
-2.8 c
-1.8 d
44.9 e |
0.0 b
-8.3 c
-7.1 d
41.0 e |
0.0 b
-5.2 c
-4.0 d
43.4 e |
0.0 b
-4.9 c
-3.8 d
44.0 e |
0.0 b
0.5 c
1.6 d
47.8 e |
0.0 b
-2.6 c
-1.6 d
46.4 e |
0.0 b
-4.9 c
-3.8 d
44.0 e |
0.0 b
-2.7 c
-1.6 d
46.4 e
55.3 f
49.5 g |
| density functional |
LSDA |
NC
NC
NC |
0.0 b
98.2 c
94.9 d
136.9 e |
0.0 b
98.2 c
94.9 d
136.9 e |
NC
NC
NC |
NC
NC
NC |
0.0 b
114.2 c
111.8 d
134.5 e |
0.0 b
119.1 c
116.9 d
139.2 e |
NC
NC
NC |
0.0 b
111.2 c
108.5 d
133.8 e |
0.0 b
120.2 c
117.7 d
139.9 e |
|
|
0.0 b
123.1 c
120.5 d
144.7 e |
0.0 b
117.4 c
114.7 d
139.5 e |
0.0 b
126.9 c
124.5 d
145.7 e |
|
NC
NC
NC |
|
| BLYP |
0.0 b
40.9 c
41.4 d
76.5 e |
0.0 b
5.2 c
5.1 d
76.3 e |
0.0 b
5.2 c
5.1 d
76.3 e |
0.0 b
18.9 c
19.4 d
90.9 e |
0.0 b
10.2 c
10.6 d
66.5 e
52.9 f
43.0 g |
0.0 b
17.2 c
17.6 d
73.4 e |
0.0 b
22.9 c
23.5 d
79.5 e |
0.0 b
6.0 c
6.5 d
65.8 e |
0.0 b
14.9 c
15.2 d
73.7 e |
0.0 b
20.1 c
20.5 d
76.5 e |
|
|
0.0 b
27.1 c
27.4 d
84.8 e |
0.0 b
17.8 c
18.3 d
77.1 e |
0.0 b
29.9 c
30.5 d
84.3 e |
|
NC
NC
NC |
|
| B1B95 |
0.0 b
74.0 c
74.2 d
82.7 e |
0.0 b
43.0 c
41.1 d
87.6 e |
0.0 b
43.0 c
41.1 d
87.6 e |
0.0 b
58.3 c
57.8 d
103.2 e |
0.0 b
48.0 c
47.6 d
73.1 e |
0.0 b
54.3 c
53.8 d
80.4 e |
0.0 b
59.7 c
59.2 d
86.1 e |
0.0 b
71.8 c
71.3 d
101.3 e |
0.0 b
52.9 c
52.1 d
81.5 e |
0.0 b
58.0 c
57.6 d
83.9 e |
|
|
0.0 b
61.5 c
60.7 d
89.5 e |
0.0 b
59.6 c
59.1 d
87.1 e |
0.0 b
66.3 c
65.8 d
91.1 e |
0.0 b
61.4 c
60.7 d |
0.0 b
59.6 c
59.1 d
87.1 e |
|
| B3LYP |
0.0 b
56.0 c
56.3 d
80.2 e |
0.0 b
19.0 c
18.6 d
82.5 e |
0.0 b
19.0 c
18.6 d
82.5 e |
0.0 b
34.8 c
35.1 d
98.9 e |
0.0 b
22.9 c
23.1 d
69.7 e |
0.0 b
30.2 c
30.4 d
77.0 e |
NC
NC
NC |
0.0 b
18.8 c
19.1 d
69.6 e |
0.0 b
27.9 c
27.9 d
77.7 e |
0.0 b
32.2 c
32.3 d
79.6 e
59.1 g |
0.0 b
34.1 c
34.2 d
83.3 e |
0.0 b
27.7 c
27.8 d
78.9 e |
0.0 b
38.3 c
38.4 d
87.2 e |
0.0 b
31.4 c
31.6 d
81.7 e |
0.0 b
42.1 c
42.5 d
88.7 e |
0.0 b
33.7 c
33.8 d
83.9 e
72.4 f
65.2 g |
0.0 b
31.4 c
31.6 d
81.7 e |
|
| B3LYPultrafine |
|
|
|
|
0.0 b
22.9 c
23.1 d
69.7 e |
|
0.0 b
83.9 e |
|
|
|
|
|
|
0.0 b
81.8 e |
|
0.0 b
33.7 c
33.9 d
83.9 e |
|
|
| B3PW91 |
0.0 b
69.6 c
70.3 d
83.8 e |
0.0 b
41.1 c
40.4 d
90.4 e |
0.0 b
41.1 c
40.4 d
90.4 e |
0.0 b
55.7 c
55.8 d
105.8 e |
0.0 b
45.1 c
45.2 d
77.0 e |
0.0 b
52.6 c
52.6 d
84.4 e |
0.0 b
58.3 c
58.4 d
90.4 e |
0.0 b
42.2 c
42.2 d
77.6 e |
0.0 b
51.3 c
51.1 d
86.0 e |
0.0 b
54.9 c
54.9 d
87.4 e |
|
|
0.0 b
59.3 c
59.2 d
93.4 e |
0.0 b
55.2 c
55.2 d
90.2 e |
0.0 b
62.9 c
63.0 d
94.9 e |
|
0.0 b
55.2 c
55.2 d
90.2 e |
|
| mPW1PW91 |
0.0 b
75.3 c
75.7 d
85.5 e |
0.0 b
47.7 c
46.6 d
93.4 e |
0.0 b
47.4 c
46.3 d
93.2 e |
0.0 b
61.7 c
61.5 d
108.6 e |
0.0 b
50.7 c
50.5 d
78.9 e |
0.0 b
58.4 c
58.1 d
86.5 e |
0.0 b
64.3 c
64.1 d
92.8 e |
0.0 b
47.7 c
47.5 d
79.6 e |
0.0 b
56.6 c
56.2 d
87.8 e |
0.0 b
60.0 c
59.8 d
88.9 e |
|
|
0.0 b
64.6 c
64.2 d
95.3 e |
0.0 b
60.6 c
60.3 d
92.1 e |
0.0 b
67.8 c
67.6 d
96.5 e |
|
0.0 b
60.5 c
60.3 d
92.1 e |
|
| M06-2X |
|
|
0.0 b
39.7 c
40.6 d
84.6 e
89.8 f
86.2 g |
|
0.0 b
39.9 c
39.4 d
63.2 e |
|
|
|
|
|
|
|
|
|
|
|
|
|
| PBEPBE |
0.0 b
69.3 c
69.4 d
88.9 e |
0.0 b
40.3 c
39.2 d
91.0 e |
0.0 b
40.3 c
39.2 d
91.0 e |
0.0 b
51.9 c
51.7 d
104.3 e |
0.0 b
45.5 c
45.2 d
80.3 e |
0.0 b
52.9 c
52.5 d
87.6 e |
0.0 b
58.0 c
57.8 d
92.9 e |
0.0 b
42.9 c
42.5 d
80.7 e |
0.0 b
52.0 c
51.5 d
89.1 e |
0.0 b
56.4 c
56.1 d
91.1 e |
|
|
0.0 b
61.2 c
60.7 d
97.7 e |
0.0 b
56.4 c
55.9 d
93.4 e |
0.0 b
64.3 c
64.1 d
98.0 e |
|
0.0 b
56.4 c
55.9 d
93.4 e |
|
| PBEPBEultrafine |
|
|
|
|
0.0 b
45.6 c
45.2 d
80.3 e |
|
|
|
|
|
|
|
|
|
|
|
|
|
| PBE1PBE |
|
|
|
|
0.0 b
55.0 c
54.6 d
80.4 e |
|
|
|
|
|
|
|
|
|
|
|
|
|
| HSEh1PBE |
|
0.0 b
48.4 c
47.1 d
93.8 e |
|
|
0.0 b
51.5 c
51.1 d
78.8 e |
|
0.0 b
65.3 c
64.9 d
92.8 e |
|
|
|
|
|
|
0.0 b
62.1 c
61.5 d
92.5 e |
|
|
|
|
| TPSSh |
|
|
|
|
0.0 b
36.1 c
36.0 d
67.8 e
56.6 f
46.7 g |
|
0.0 b
49.4 c
49.4 d
81.3 e
66.2 f
57.4 g |
|
|
0.0 b
44.9 c
44.8 d
77.4 e
65.4 f
56.5 g |
|
|
|
0.0 b
46.3 c
46.1 d
81.3 e
70.1 f
61.6 g |
|
|
|
|
| wB97X-D |
|
|
0.0 b
41.2 c
39.1 d
87.5 e
91.1 f
86.2 g |
|
0.0 b
42.8 c
41.7 d
72.7 e
59.9 f
52.8 g |
|
0.0 b
55.5 c
54.3 d
86.0 e
69.4 f
63.2 g |
|
0.0 b
47.4 c
45.9 d
80.8 e
71.6 f
65.3 g |
|
|
0.0 b
46.2 c
44.9 d
80.4 e
72.2 f
66.1 g |
0.0 b
55.5 c
54.3 d
86.0 e
69.4 f
63.2 g |
0.0 b
50.4 c
49.0 d
84.1 e
72.9 f
67.1 g |
|
0.0 b
51.7 c
50.2 d
85.2 e
73.1 f
67.2 g |
|
|
| B97D3 |
|
0.0 b
16.9 c
15.1 d
82.4 e
78.2 f
69.9 g |
|
|
0.0 b
22.1 c
20.8 d
69.3 e
55.5 f
45.3 g |
|
0.0 b
35.3 c
34.1 d
82.6 e
65.4 f
56.4 g |
|
0.0 b
28.9 c
27.3 d
78.2 e
67.8 f
58.8 g |
|
0.0 b
33.9 c
32.4 d
82.3 e
70.1 f
61.5 g |
0.0 b
27.5 c
26.1 d
79.1 e
68.5 f
59.7 g |
|
0.0 b
31.2 c
29.9 d
81.0 e
69.8 f
61.4 g |
|
0.0 b
33.2 c
31.7 d
82.7 e
70.2 f
61.7 g |
|
|
| |
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
cc-pV(T+d)Z |
daug-cc-pVTZ |
|---|
| Moller Plesset perturbation |
MP2 |
0.0 b
-78.9 c
-78.2 d
-40.6 e |
0.0 b
-40.0 c
-40.4 d
26.0 e |
0.0 b
-40.0 c
-40.4 d
26.0 e |
0.0 b
-26.9 c
-26.4 d
38.0 e |
0.0 b
18.1 c
17.7 d
48.9 e
62.5 f
53.3 g |
0.0 b
23.2 c
22.8 d
53.7 e |
|
0.0 b
21.7 c
20.8 d
52.6 e
66.8 f
58.2 g |
0.0 b
27.7 c
26.9 d
57.7 e |
0.0 b
40.1 c
38.4 d
66.9 e |
|
NC
NC
NC |
0.0 b
198.2 c
28.5 d
63.2 e |
0.0 b
45.2 c
43.4 d
75.5 e |
|
0.0 b
49.8 c
47.3 d |
NC
NC
NC |
|
| MP2=FULL |
|
|
|
|
0.0 b
19.3 c
18.8 d
49.4 e |
0.0 b
24.3 c
23.9 d
54.1 e |
0.0 b
35.2 c
34.8 d
65.3 e |
0.0 b
23.5 c
22.5 d
53.1 e |
0.0 b
29.6 c
28.8 d
58.3 e |
|
|
|
|
0.0 b
48.8 c
47.4 d |
|
|
|
|
| MP3 |
|
|
|
|
0.0 b
19.5 c
19.7 d
55.0 e |
|
|
|
|
|
|
|
|
|
|
|
|
|
| MP3=FULL |
|
|
|
|
0.0 b
20.4 c
20.6 d
55.2 e
50.5 f
43.9 g |
|
0.0 b
34.7 c
35.2 d
69.7 e |
|
|
|
|
|
|
|
|
|
|
|
| B2PLYP |
|
|
|
|
0.0 b
16.2 c
16.2 d
58.7 e |
|
|
|
|
|
|
|
|
NC
NC
NC |
|
|
|
|
| B2PLYP=FULLultrafine |
|
|
|
|
0.0 b
16.6 c
16.6 d
58.9 e |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Configuration interaction |
CID |
|
|
|
|
0.0 b
13.7 c
14.2 d
46.5 e |
|
|
|
|
|
|
|
|
|
|
|
|
|
| CISD |
|
|
|
|
0.0 b
12.0 c
12.4 d
45.6 e |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
cc-pV(T+d)Z |
daug-cc-pVTZ |
|---|
| Quadratic configuration interaction |
QCISD |
|
0.0 b
-33.6 c
-33.6 d
38.9 e |
|
|
|
0.0 b
44.8 e |
0.0 b
54.4 e |
|
0.0 b
45.1 e |
|
|
|
|
|
|
|
|
|
| Coupled Cluster |
CCD |
|
|
|
|
0.0 b
6.5 c
6.7 d
43.1 e |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
cc-pV(T+d)Z |
daug-cc-pVTZ |
|---|