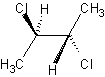
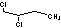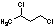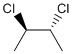Methods with standard basis sets
|
|
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
daug-cc-pVTZ |
| hartree fock |
HF |
0.0 a
-0.5 b
-5.7 c
-7.2 e |
0.0 a
-9.5 b
-14.1 c
-22.3 e |
0.0 a
-5.3 b
-9.8 c
-13.2 e |
0.0 a
-3.8 b
-10.8 c
-13.7 e |
0.0 a
-0.4 b
-6.0 c
-4.7 e |
0.0 a
-0.9 b
-6.5 c
-5.5 e |
0.0 a
-0.6 b
-6.5 c
-5.1 e |
0.0 a
-0.6 b
-6.3 c
-4.8 e |
0.0 a
-1.3 b
-7.0 c
-5.9 e |
0.0 a
-1.2 b
-6.2 c
-5.4 e |
0.0 a
-1.9 b
-6.9 c
-6.7 e |
0.0 a
-1.2 b
-6.6 c
-5.5 e |
0.0 a
-3.5 b
-8.2 c
-8.9 e |
0.0 a
-1.1 b
-6.1 c
-5.0 e |
0.0 a
-1.7 b
-7.1 c
-6.9 e |
0.0 a
-0.5 b
-5.9 c
-4.4 e |
0.0 a
-0.5 b
-5.9 c
-4.3 e |
| density functional |
LSDA |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
NC
NC
NC |
NC
NC
NC |
NC
NC
NC |
|
|
| BLYP |
0.0 a
-6.1 b
-7.7 c
-14.3 e |
0.0 a
-14.7 b
-14.9 c
-28.2 e |
|
0.0 a
-9.5 b
-12.3 c
-20.6 e |
0.0 a
-5.9 b
-8.7 c
-12.4 e |
0.0 a
-6.1 b
-8.9 c
-12.8 e |
0.0 a
-5.4 b
-8.8 c
-12.0 e |
|
0.0 a
-6.0 b
-9.3 c
-12.8 e |
0.0 a
-7.1 b
-9.3 c
-13.8 e |
|
|
0.0 a
-8.1 b
-10.0 c
-14.9 e |
0.0 a
-6.1 b
-8.5 c
-12.0 e |
0.0 a
-5.7 b
-8.7 c
-11.9 e |
|
|
| B1B95 |
0.0 a
-4.9 b
-7.8 c
-13.1 e |
|
0.0 a
-14.3 b
-15.2 c
-26.8 e |
0.0 a
-10.8 b
-14.5 c
-23.5 e |
0.0 a
-7.7 b
-10.3 c
-15.5 e |
0.0 a
-8.3 b
-10.7 c
-16.3 e |
0.0 a
-8.0 b
-10.9 c
-16.1 e |
0.0 a
-7.8 b
-10.8 c
-16.0 e |
0.0 a
-8.7 b
-11.5 c
-17.2 e |
0.0 a
-8.7 b
-10.4 c
-16.4 e |
|
|
0.0 a
-10.4 b
-12.0 c
-18.5 e |
0.0 a
-8.5 b
-10.4 c
-16.3 e |
0.0 a
-8.9 b
-11.0 c
-17.0 e |
|
|
| B3LYP |
0.0 a
-5.2 b
-7.8 c
-13.5 e |
0.0 a
-14.6 b
-16.0 c
-28.8 e |
0.0 a
-9.6 b
-11.9 c
-19.3 e |
0.0 a
-8.6 b
-12.6 c
-19.9 e |
0.0 a
-5.2 b
-8.7 c
-11.8 e |
0.0 a
-5.6 b
-9.0 c
-12.4 e |
0.0 a
-5.1 b
-9.0 c
-12.0 e |
0.0 a
-5.0 b
-8.8 c
-11.7 e |
0.0 a
-5.9 b
-9.6 c
-12.9 e |
0.0 a
-6.4 b
-9.1 c
-13.1 e |
0.0 a
-6.4 b
-9.4 c
-13.2 e |
0.0 a
-6.2 b
-9.4 c
-12.8 e |
0.0 a
-7.7 b
-10.2 c
-14.7 e |
0.0 a
-5.8 b
-8.7 c
-11.9 e |
0.0 a
-5.5 b
-8.9 c
-12.1 e |
0.0 a
-5.2 b
-8.5 c
-11.3 e |
|
| B3LYPultrafine |
|
|
|
|
0.0 a
-5.2 b
-8.7 c
-11.9 e |
|
|
|
|
|
|
|
|
|
|
0.0 a
-5.2 b
-8.5 c
-11.3 e |
|
| B3PW91 |
0.0 a
-3.6 b
-7.0 c
-11.3 e |
0.0 a
-15.7 b
-17.2 c
-31.1 e |
0.0 a
-10.7 b
-12.8 c
-21.3 e |
0.0 a
-8.7 b
-12.9 c
-20.4 e |
0.0 a
-5.3 b
-8.7 c
-12.1 e |
0.0 a
-5.9 b
-9.2 c
-12.9 e |
0.0 a
-5.8 b
-9.4 c
-12.9 e |
0.0 a
-5.7 b
-9.2 c
-12.6 e |
0.0 a
-6.7 b
-10.1 c
-14.1 e |
0.0 a
-6.2 b
-8.8 c
-12.9 e |
|
|
0.0 a
-7.9 b
-10.4 c
-15.3 e |
0.0 a
-6.0 b
-8.6 c
-12.2 e |
0.0 a
-6.4 b
-9.4 c
-13.4 e |
|
|
| mPW1PW91 |
0.0 a
-4.3 b
-7.6 c
-12.4 e |
0.0 a
-16.9 b
-18.3 c
-33.1 e |
0.0 a
-11.9 b
-13.9 c
-23.2 e |
0.0 a
-9.5 b
-13.6 c
-21.6 e |
0.0 a
-6.1 b
-9.3 c
-13.5 e |
0.0 a
-6.7 b
-9.8 c
-14.3 e |
0.0 a
-6.5 b
-9.9 c
-14.2 e |
0.0 a
-9.7 c
-13.8 e |
0.0 a
-7.3 b
238.3 c
-15.2 e |
0.0 a
-7.0 b
-9.5 c
-14.1 e |
|
|
0.0 a
-8.8 b
-11.1 c
-16.9 e |
0.0 a
-6.8 b
-9.4 c
-13.5 e |
0.0 a
-7.2 b
-10.1 c
-14.8 e |
|
|
| M06-2X |
|
|
0.0 a
-18.7 b
-20.7 c
-31.8 d
-30.3 e |
|
0.0 a
-11.5 b
-13.8 c
-21.6 e |
|
|
|
|
|
|
|
|
|
|
|
|
| PBEPBE |
0.0 a
-6.7 b
-8.6 c
-15.5 e |
0.0 a
-19.0 b
-18.8 c
-35.2 e |
0.0 a
-13.8 b
-14.6 c
-25.4 e |
0.0 a
-11.9 b
-14.6 c
-24.6 e |
0.0 a
-8.3 b
-10.6 c
-16.2 e |
0.0 a
-8.8 b
-11.0 c
-16.9 e |
0.0 a
-8.4 b
-11.0 c
-16.7 e |
0.0 a
-8.3 b
-10.8 c
-16.3 e |
0.0 a
-9.1 b
-11.6 c
-17.7 e |
0.0 a
-9.4 b
-10.8 c
-17.2 e |
|
|
0.0 a
-10.9 b
-12.2 c
-19.2 e |
0.0 a
-9.0 b
-10.6 c
-16.4 e |
0.0 a
-8.9 b
-11.0 c
-16.9 e |
|
|
| PBEPBEultrafine |
|
|
|
|
0.0 a
-8.2 b
-10.4 c
-16.1 e |
|
|
|
|
|
|
|
|
|
|
|
|
| PBE1PBE |
|
|
|
|
0.0 a
-7.1 b
-10.1 c
-14.8 e |
|
|
|
|
|
|
|
|
|
|
|
|
| HSEh1PBE |
|
0.0 a
-18.3 b
-19.2 c
-35.0 e |
|
|
0.0 a
-7.4 b
-10.2 c
-15.3 e |
|
0.0 a
-7.7 b
-10.8 c
-16.0 e |
|
|
|
|
|
|
0.0 a
-8.0 b
-10.2 c
-15.5 e |
|
|
|
| TPSSh |
|
|
|
|
0.0 a
-6.7 b
-9.3 c
-14.0 e |
|
0.0 a
-6.9 b
-9.7 c
-14.4 e |
|
|
0.0 a
-7.6 b
-9.4 c
-14.6 e |
|
|
|
0.0 a
-7.2 b
-9.1 c
-13.9 e |
|
|
|
| wB97X-D |
|
|
0.0 a
-11.1 b
-13.8 c
-22.4 e |
|
0.0 a
-6.4 b
-10.3 c
-14.4 e |
|
0.0 a
-6.7 b
-10.9 c
-15.2 e |
|
0.0 a
-7.8 b
-11.7 c
-17.0 e |
|
|
0.0 a
-7.5 b
-11.1 c
-15.6 e |
0.0 a
-6.7 b
-10.9 c
-15.2 e |
0.0 a
-6.9 b
-10.7 c
-14.6 e |
|
0.0 a
-6.4 b
-10.4 c
-14.0 e |
|
| B97D3 |
|
0.0 a
-20.6 b
-19.9 c
-36.9 e |
|
|
0.0 a
-11.2 b
-12.7 c
-20.0 e |
|
0.0 a
-11.2 b
-13.1 c
-20.4 e |
|
0.0 a
-11.8 b
-13.6 c
-21.3 e |
|
0.0 a
-12.5 b
-13.4 c
-23.4 d
-21.5 e |
0.0 a
-12.0 b
-13.3 c
-22.9 d
-20.8 e |
|
0.0 a
-11.7 b
-12.7 c
-20.0 e |
|
0.0 a
-11.1 b
-12.5 c
-19.4 e |
|
| |
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
daug-cc-pVTZ |
|---|
| Moller Plesset perturbation |
MP2 |
0.0 a
-3.0 b
-7.0 c
-11.1 e |
0.0 a
-14.3 b
-16.5 c
-29.8 e |
0.0 a
-14.6 b
-16.4 c
-28.5 e |
0.0 a
-10.2 b
-14.3 c
-23.7 e |
0.0 a
-11.7 b
-14.1 c
-23.5 e |
0.0 a
-11.7 b
-13.9 c
-23.3 e |
|
0.0 a
-13.6 b
-15.6 c
-26.2 e |
0.0 a
-13.6 b
-15.1 c
-25.6 e |
0.0 a
-15.2 b
-15.9 c
-28.0 e |
|
0.0 a
-15.6 b
-16.3 c
-27.9 e |
0.0 a
-14.2 b
-15.2 c
-25.9 e |
0.0 a
-15.4 c
-26.9 e |
|
|
|
| MP2=FULL |
|
|
|
|
0.0 a
-12.3 b
-14.6 c
-24.5 e |
|
|
0.0 a
-14.2 b
-16.1 c
-27.1 e |
0.0 a
-14.2 b
-15.6 c
-26.5 e |
|
|
|
|
|
|
|
|
| MP3 |
|
|
|
|
0.0 a
-8.1 b
-11.4 c
-17.3 e |
|
|
|
|
|
|
|
|
|
|
|
|
| MP3=FULL |
|
|
|
|
0.0 a
-8.6 b
-11.8 c
-18.1 e |
|
0.0 a
-10.0 b
-13.1 c
-20.0 e |
|
|
|
|
|
|
|
|
|
|
| B2PLYP |
|
|
|
|
0.0 a
-7.5 b
-10.7 c
-16.0 e |
|
|
|
|
|
|
|
|
0.0 a
-9.1 b
-11.2 c
-17.5 e |
|
|
|
| B2PLYP=FULLultrafine |
|
|
|
|
NC
NC
NC |
|
|
|
|
|
|
|
|
|
|
|
|
| |
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
daug-cc-pVTZ |
|---|
| Quadratic configuration interaction |
QCISD |
|
0.0 a
-15.5 c |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
STO-3G |
3-21G |
3-21G* |
6-31G |
6-31G* |
6-31G** |
6-31+G** |
6-311G* |
6-311G** |
6-31G(2df,p) |
6-311+G(3df,2p) |
TZVP |
cc-pVDZ |
cc-pVTZ |
aug-cc-pVDZ |
aug-cc-pVTZ |
daug-cc-pVTZ |
|---|