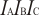Geometric Data

Point Group D3h
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
| Description |
Value |
unc. |
Connectivity |
Reference |
Comment |
| Atom 1 |
Atom 2 |
Atom 3 |
Atom 4 |
Cartesians
Atom - Atom Distances 
Distances in Å
Calculated geometries
for BCl
3+ (Boron Trichloride cation).
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
Connectivity
| Atom 1 |
Atom 2 |
| B1 |
Cl2 |
| B1 |
Cl3 |
| B1 |
Cl4 |
Dipole, Quadrupole and Polarizability
Electric dipole moment 
| State |
Config |
State description |
Conf description |
Exp. min. |
Dipole (Debye) |
Reference |
comment |
Point Group |
Components |
| x |
y |
z |
total |
dipole |
quadrupole |
| 1 |
1 |
2A2' |
D3h |
False |
|
|
|
|
|
|
D3h |
0 |
1 |
Experimental dipole measurement abbreviations: MW microwave; DT Dielectric with Temperature variation; DR Indirect (usually an upper limit); MB Molecular beam
Calculated electric dipole moments for
BCl
3+ (Boron Trichloride cation).
Electric quadrupole moment 
| State |
Config |
State description |
Conf description |
Exp. min. |
Quadrupole (D Å) |
Reference |
comment |
Point Group |
Components |
| xx |
yy |
zz |
dipole |
quadrupole |
| 1 |
1 |
2A2' |
D3h |
False |
|
|
|
|
|
D3h |
0 |
1 |
Calculated electric quadrupole moments for
BCl
3+ (Boron Trichloride cation).