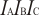Geometric Data
Point Group C2
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
| Description |
Value |
unc. |
Connectivity |
Reference |
Comment |
| Atom 1 |
Atom 2 |
Atom 3 |
Atom 4 |
Cartesians
Atom - Atom Distances 
Distances in Å
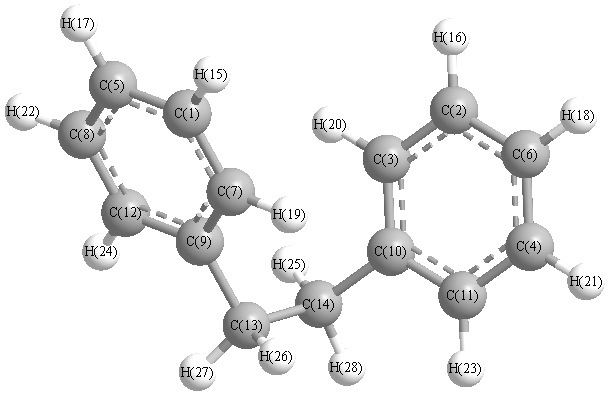
Calculated geometries
for C
14H
14 (Bibenzyl).
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| C:C |
12 |
| C-C |
3 |
| H-C |
14 |
Connectivity
| Atom 1 |
Atom 2 |
| C1 |
C5 |
| C1 |
C7 |
| C1 |
H15 |
| C2 |
C3 |
| C2 |
C6 |
| C2 |
H16 |
| C3 |
C10 |
| C3 |
H20 |
| C4 |
C6 |
| C4 |
C11 |
| C4 |
H21 |
| C5 |
C1 |
| C5 |
C8 |
| C5 |
H17 |
| C6 |
H18 |
| C7 |
C7 |
| C7 |
H19 |
| C8 |
C12 |
| C8 |
H22 |
| C9 |
C12 |
| C9 |
C13 |
| C10 |
C11 |
| C10 |
C14 |
| C11 |
H23 |
| C12 |
H24 |
| C13 |
H26 |
| C13 |
H27 |
| C14 |
H25 |
| C14 |
H28 |